This is the second of a three-part series on anticholinergic burden. You can read part 1 here.
ON paper, anticholinergic burden scales for medicines could be so great.
To prevent falls and confusion, their earnest promise is alluring – the ability to calculate how adversely anticholinergic a drug regime may be. This is especially considering the blurb within one anticholinergic scale that states “each definite anticholinergic may increase the risk of cognitive impairment by 46% over 6 years” and “for each one point increase in the total score, a decline in Mini Mental State Examination score of 0.33 points over 2 years has been suggested”.
Powerful stuff.
Enticed accordingly when I became a consultant, I frequently tried calculating the anticholinergic burden of patients’ medications, scouring the published scales I mentioned in part 1 of this series. The aim was to add anticholinergic scores to my letters to referring GPs – lending quantitative weight to any deprescribing options proposed.
Sadly though, this practice soon fizzled.
Things just weren’t adding up – the published scales I had in hand were correlating poorly among themselves (here, here and here). In the end, I realised straightforward calculation of anticholinergic burden scores is hampered by several barriers, including:
- individual drugs being granted differing scores between rival scales;
- not all medications patients take will necessarily be found on published scales;
- some scales are hard to access (ie, cannot be freely downloaded, cannot be found in an app);
- difficulty understanding how some drugs could have been deemed mildly anticholinergic – such as warfarin and frusemide – thus provoking doubts about the veracity of the entire concept;
- a lack of consideration of potentially dangerous yet non-anticholinergic drugs – those with links to falls and confusion (ie, sedatives); and
- drug dosages not being taken into account when calculating burden or risk.
So, a fair bit of concern!
However, before delving deeper into these problems, let’s walk through how exactly things got messy (dating back a few years) and set the scene.
The backstory of anticholinergic scales
Some of the original published anticholinergic scales (yes, plural!) may be known to you. They include:
- the ABC (Anticholinergic Burden Classification, 2006);
- the ADS (Anticholinergic Drug Scale, 2006);
- the ARS (Anticholinergic Risk Scale, 2008); and
- the ACB (Anticholinergic Cognitive Burden, 2008); updated scale in 2012.
As shown, most scales surfaced within the past 10–15 years. However, the underlying concept – that some unexpected drugs may have the latent ability to block muscarinic receptors in the nervous system – originated around 40 years ago.
In 1980, two psychiatrists in Baltimore published a laboratory technique whereby drugs – dissolved in a solution – could have their relative anticholinergic abilities measured (see Figure 1).
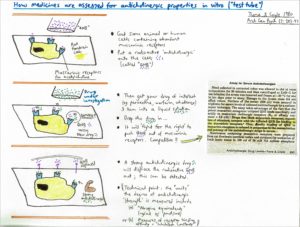
Figure 1. In vitro assessment of drugs for anticholinergic properties. Tune and Coyle 1980 Arch Gen Psych 37: 293-297
In short, they gathered some pre-prepared brain cells from rats – cells known to have plentiful muscarinic receptors on their surfaces. Onto these, they drizzled a pre-prepared radioactive solution of a known antimuscarinic called QNB, which naturally then blocked up the receptors. But because anticholinergic drugs compete for occupancy of the receptors, if they then dropped a liquid solution of a drug they wanted to study onto the brain cells, any medication heavily out-competing and displacing the QNB would be assumed to be a champion anticholinergic – a bit like if someone strongly holding a prized object (QNB clasping the receptor) is defeated by someone arriving with an even stronger grip (ie, victory to the new drug being studied). Strong anticholinergics such as atropine or oxybutynin may come along and bump off the QNB with relative ease – and this can be measured.
Using this technique in 1992, Tune and colleagues then published a famous list (Table 1) depicting the in vitro anticholinergic activity of 25 commonly used medications. Every drug they studied was adjusted to the exact same concentration (10-8 M). It was in this laboratory study that surprising drugs such as frusemide, digoxin, nifedipine and warfarin were first flagged as mildly anticholinergic.
That said, an obvious issue with this study was that it only assessed 25 drugs from the entire pharmacopoeia. Subsequent critics also noted that all 25 drugs were made up to the same concentration, stating that this single concentration “may not be clinically relevant for many of the drugs studied,” implying a lack of clinical applicability.

Table 1. Anticholinergic drug levels in 25 medications. Tune et al, Am J Psych 1992; 149: 1393-1394
Notwithstanding, outside this 1992 article, only a handful of similar studies were performed (in the 1980s and 1990s); some of these explored the anticholinergic effects of older antihistamines and tricyclic antidepressants.
Interestingly, it wasn’t then until 2008 that much else surfaced regarding the in vitro anticholinergic activity of medicines – when a larger laboratory study by Chew and colleagues looked at 107 drugs made up at arguably more relevant physiological concentrations. They found that certain commonly used drugs could be somewhat anticholinergic depending on dose – including ranitidine, olanzapine, paroxetine, quetiapine and mirtazapine. They also found that some medicines deemed to be anticholinergic in 1992 were not actually so within their study – these included warfarin, nifedipine, codeine, frusemide and digoxin. And to add further difficulty, the 2008 analysis measured the anticholinergic activity in different units from Tune and colleagues’ article (pmol/mL rather than ng/mL). This makes comparison of the two lists further complicated.
But what is perhaps most noteworthy here is the fact that this larger study by Chew in 2008 – looking at 107 rather than only 25 drugs – was published contemporaneously with (or even after) many of the original anticholinergic scales (circa 2006–2008).
This means that researchers involved in developing early scales (such as the ADS, 2006) likely didn’t have access to the 2008 in vitro findings. Accordingly, they may have believed, for example, that warfarin is mildly anticholinergic, given it was said to be so in 1992 – even though it was thought to have no anticholinergic activity later in 2008.
The birth of the original anticholinergic scales
So, in this context, how did the original scales arise? If there was such a dearth of reliable, consistent laboratory studies on anticholinergic drug activity, how did the authors of such scales “know” that listed drugs are anticholinergic or otherwise?
In short, the (perhaps imperfect) answer is that this was decided via a process of expert consensus (here, here and here). Simplistically, anticholinergic scales are devised as follows:
- a master list of drugs is written down – for example, this may be the most popularly prescribed medicines in that country;
- a group of experts is assembled – typically comprising a doctor, pharmacologist and scientist – and each person is asked to score each medication on how anticholinergic they think it is: namely its ability to cause problems such as confusion, dry mouth or dizziness. Subsequently, the group determines how much they agree or disagree, per drug; and
- the panel members may use literature to help them determine how anticholinergic they believe each drug may be – that is, pharmaceutical texts plus whatever in vitro studies are available (albeit with the vagaries and rarity of such data).
What are the problems we’re left with today?
The current vexed reality of using the well known anticholinergic scales includes at least the following four issues:
- First, there are nearly 20 common drugs for which the anticholinergic score (0, 1, 2 or 3) varies across published scales (Figure 2). For example, quetiapine, colchicine and ranitidine may have wildly differing scores between 1 and 3. These discrepancies erode faith in the exercise and may cause frustrated doctors to abandon it. One does not intuitively know which scale is the best or most accurate.

Figure 2. Anticholinergic score variations in different published scales. Salahudeen et al; BMX Geriatrics 2015; 15:31
- Second, regarding some drugs given a score of 1, it is sometimes genuinely difficult to comprehend how this came to be – for example, do doctors really buy in to the idea that drugs such as nifedipine, frusemide, warfarin and prednisolone are somehow mildly anticholinergic? Classically, anticholinergics are said to cause constipation and dry mouth – and indeed so too do calcium-channel blockers and loop diuretics. But this begs the question: is that actually because of an occult anticholinergic effect? Can’t xerostomia on frusemide simply relate to dehydration from diuresis? The genesis of the largest scale (ARS, listing 117 anticholinergic drugs) involved three experts judging the likelihood of medications causing one of six symptoms: falls, xerostomia, dry eyes, constipation, confusion and dizziness. Yet, one wonders if these apparently anticholinergic symptoms are particularly specific. Couldn’t dizziness instead relate to hypoglycaemia, seizures or hypotension? With such questions nagging, we may turn to the laboratory – but again, given concerns about the quality and number of studies on the test tube anticholinergic effect of drugs today, can we really be sure that in vitro lists are reliable? This is even further complicated by the fact that studies years ago suggested that whole serum from patients with acute illness may have, in itself, strong anticholinergic activity, even if free of medications (here and here, but countered here and here). This implies that the body may endogenously produce anticholinergic molecules when we are sick, irrespective of what we do to medication lists. And via meta-analysis, others have further found a questionable correlation between the serum anticholinergic activity (in vitro) and negative cognitive outcomes – another area of uncertainty. Complex!
- Third, doctors may find that a drug they are reviewing is absent from the scale in front of them. None of the older scales comment on the anticholinergic tendencies of newer drugs such as apixaban. And then there is the concern that, for example, the ACB lists diazepam and alprazolam as scoring 1, but there is no mention of other benzodiazepines, which makes one wonder what to do if, say, scrutinising a chart containing oxazepam.
- Fourth, at its core, the chief rallying cry of the anticholinergic burden concept is to say: “these drugs may impair the patient’s cognition, mobility and balance”. Accordingly, what then do we make of falls-inducing drugs such as levetiracetam or pregabalin? These soporific drugs are associated with drowsiness and falls – yet have no particular anticholinergic properties according to the various scales. Furthermore, isn’t the dose of the offending drug important? The four scales listed earlier do not incorporate dosages to grade the severity of the overall anticholinergic burden, but doctors would expect 50 mg of amitryptiline to elicit greater risk than 10 mg. It is worth noting in this context that Professor Sarah Hilmer and colleagues in Sydney pioneered the development of a unique, clinically incisive scale named the Drug Burden Index (DBI). Its core feature, setting it nicely apart from others, is that it includes anticholinergics but also sedatives that may otherwise be missed – such as pregabalin, benzodiazepines and antiepileptics. The DBI also adjusts the risk score for each of a patient’s medications by the dose. If groups of older people are compared, those with high DBI scores commonly fare worse (reviewed here and here); for example, they associate with triple the risk of incident delirium requiring admission, and more than 50% greater chance of being either hospitalised with falls or readmitted to hospital if socially isolated. However, problems exist regarding the use of the DBI, acknowledged by its founders (here and here). For instance, it requires knowledge of the minimum licenced dose for every drug – something that can be surprisingly hard to discern, and sometimes varies depending on the drug’s indication. Clinicians also need a DBI calculator, but this isn’t readily available at the time of writing.
In conclusion
The popular concept of anticholinergic burden certainly sounds authoritative – but using anticholinergic scales in practice is nowhere near as easy as one hopes. Such a discovery can be deflating for doctors seeking “wins” for their patients, seeking to prevent iatrogenesis.
Nevertheless, I’d argue that analysis of real-life problems encountered can sometimes set us free on the path to positive solutions. To be continued next week, with some proposals for moving forward.
Dr Toby Commerford is a consultant geriatrician at Royal Adelaide Hospital, is course coordinator for geriatrics at the University of Adelaide’s Rural School, and practises remote and rural outreaches to Port Augusta and Murray Mallee. He is also the lead singer in a rock band.
The statements or opinions expressed in this article reflect the views of the authors and do not represent the official policy of the AMA, the MJA or InSight+ unless so stated.

 more_vert
more_vert
Thanks a ton for the article and briefs about how it is all connected. I have been trying to make sense of this area for the past 4 weeks. But wasnt able to figure out the connection between scales and values from the physiological studies
Thank you so much for this article. I found this supremely insightful. I am actually preparing a grand round presentation on anticholinergic toxicity and I always wondered how frusemide and warfarin etc could be an anticholinergic.
Again, many thanks for your hard work reviewing the literature to date. I have learned a lot.