UNFORTUNATELY, I recently realised I’m not as clever as I’d hoped. “You must be so smart to be a doctor”, people say. And yet, it has taken me about 20 years to grasp something quite seminal.
Think about all those times you may – or may not – have partaken in drunken karaoke or dancing (disclaimer: guilty). Consider 1980s hairstyles (not guilty). Consider many of today’s political “promises” (hmmm). What do all of these have in common?
In summary, the lesson that “some things are better in theory than in reality”.
That seems perhaps obvious; yet, it took me a surprisingly long time to deeply grasp the relevance of this for medical work – specifically when facing prevailing medical practices or traditions.
I think back to meeting dizzy patients in the past. Like an anxious sheep, I followed the traditional paradigm, strenuously trying to discern what patients “meant” by their presenting complaint. Endeavouring to fit them into either the “vertigo” or “lightheaded” camp, I’d find myself saying embarrassing things such as, “so you’re saying it’s more ‘spinny’ than anything else”, when the patient truthfully couldn’t elaborate beyond “dizzy” or “giddy”. I’ve written on this before (here and here).
Then there is neurological “sensory testing”. Theory is definitely better than reality in this realm. For example, you might think, “let’s rigorously assess the dermatomes,” but you may need to reconfirm the boundaries via a reference diagram. So, you look online – and then get sucked into a vacuum, peering at pictures where the dermatomes differ from one image to the next. Does the big toe’s sensation feed into L4? Or is it L5? You may zealously think, “let’s test all modalities”. But you then spend time wondering where to find Neuro Tips, a tuning fork and something cold or hot (in the past I’ve tried ice cubes, but the patient gets blobs of water smeared on them as they melt). And in the end, patients frequently tell you they can feel something, even when you aren’t touching them.
The list goes on. Dogma regarding tight sugar control in type 2 diabetes conferring cardiovascular advantage was abruptly thrown into doubt after the ill-fated ACCORD study of 2008. Then there is the common clinical assumption that delirium is invariably triggered by urinary tract infections (UTIs). However, in my experience, UTI is overdiagnosed; also, such labelling could cause us to miss alternate sources of fever – from epidural abscesses through to neuroleptic malignant syndrome.
In short, we have an intriguing backstory – namely, that the fabric of “well meaning” clinical constructs may be frayed, and that some concepts may become unwieldy, dubious or “full of holes” – rather than helpful, as they were doubtless intended at the outset.
A cracking example of this is the concept of the “anticholinergic burden” of medications.
Coming up
In this and the following two articles, I want to share my journey trying to understand and use anticholinergic burden scales – things that seem helpful on paper, yet prove otherwise in reality, at least sometimes.
The reasons for sharing this are threefold:
- First, I want to highlight and validate something important that happens to doctors – possibly to all of us. Using the concept of “anticholinergic burden” as an exemplar, if you have scratched your head for hours trying to study something seemingly important, yet find the nut is impossible to crack – it is possible that the concept has some holes in it, rather than your own cerebrum being the sole problem. Real life storytelling about how medical constructs may harbour their own imperfections (however established they may be) strikes me as essential if we are to unravel some of the problems we face practising medicine.
- Second, I hope to provide some specific educational pearls on the anticholinergic topic – both from the theory side (this article) and the reality side (part 2).
- Third, in the spirit of not simply complaining without a solution, I hope to springboard to proposing ideas for the future – in this case, proposing solutions to make bedside calculation of anticholinergic burden more straightforward (part 3).
Setting the scene
This statistic still amazes me to this day – a smoking-gun of a statistic:
20–30% of all admissions to hospital in older people in Australia are thought to be somehow related to medication.
In modern parlance, this is epic. It also validates the mantra “it’s the medication until proven otherwise” when encountering clinical problems. It also likely means we expend substantial energy tweaking medications hoping to avoid harming patients. Yet it isn’t always easy to tweak pills. And anticholinergics may be one class of these: let’s explore this further.
Back in the day and the concept of anticholinergic burden
As a registrar in 2008, I remember the first time I heard of the concept of the anticholinergic burden of individual medications. You may have come across what I mean: lists of drugs, each variably possessing an apparent ability to block muscarinic receptors in the brain and periphery – whether deliberately or just inadvertently. How badly medicines are deemed anticholinergic is typically given a score from 0 to 3:
- To start, drugs with no link whatsoever to the blockade of acetylcholine as a synaptic neurotransmitter are typically scored a 0.
- In contrast, medicines heavily antagonising muscarinic receptors – such as atropine or amitryptiline – score a 3. These big hitters can trigger the famous “blind as a bat, mad as a hatter” anticholinergic syndrome made memorable by its literary panache. Interestingly, acute administration of scopolamine – a powerful anticholinergic – is said to trigger severe cognitive disturbance to the point that it is reportedly used by criminals to drug victims – hence its nickname, “Devil’s breath”.
- Meanwhile, medications with merely “test-tube” anticholinergic properties typically score a 1. Finally, drugs with moderate muscarinic-blocking effects or anticholinergic symptoms will be scored as 2.
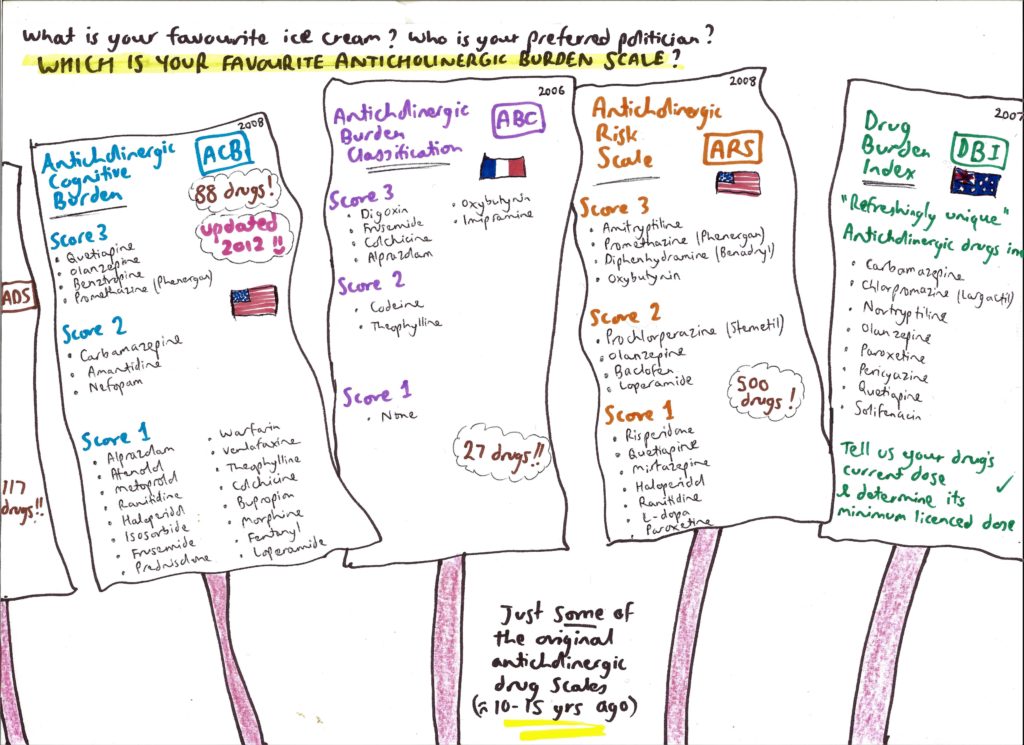
Figure 1: An overview of anticholinergic burden scales
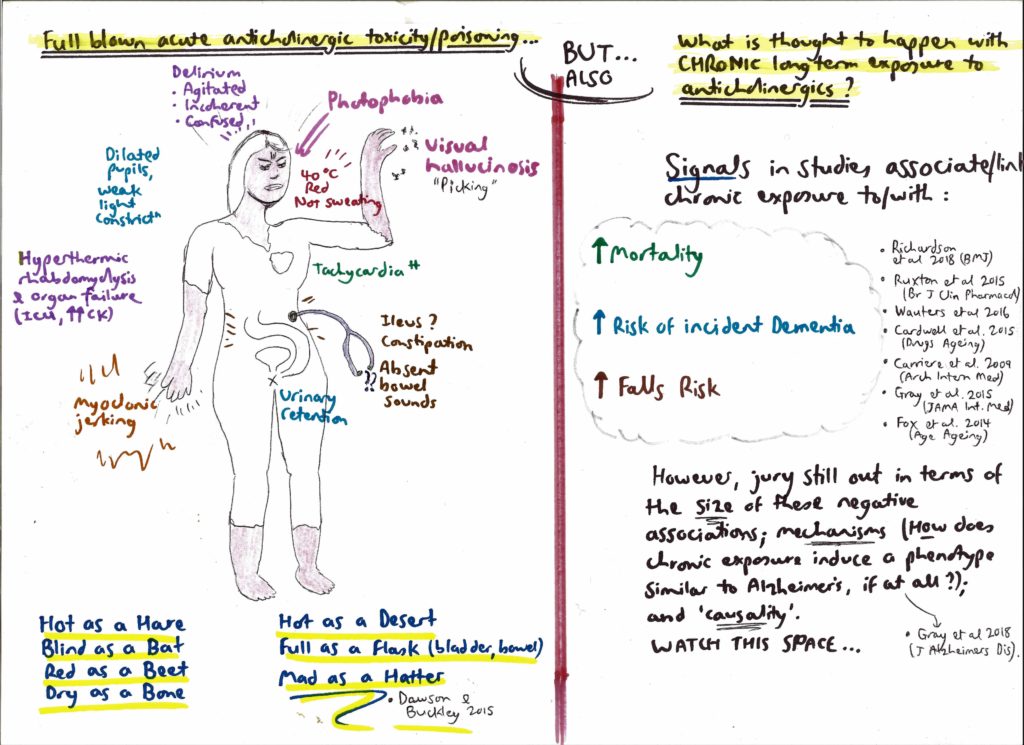
Figure 2: Anticholinergic syndrome
Anticholinergics have a bad name
The central purpose of bothering to understand the hidden or overt anticholinergic burden of medications is to stimulate doctors to action, namely to shine a suspicious light on listed drugs (“query deprescribe”). This is because anticholinergic states are thought to encourage bad outcomes – especially falls and confusion in older people. A snapshot of relevant literature includes the following:
- an Australian meta-analysis, published in 2015, looked at the effects of anticholinergic medicines on the risk of falls, cognitive impairment and mortality in older people. Chronic exposure to anticholinergic drugs broadly increased the odds of patients developing cognitive decline by 45%, and falls risk was variably increased, depending on the exact drug;
- at least two studies have shown that chronic use of anticholinergic medication is associated with reduced ability to perform daily living tasks independently (eg, shopping, bill paying); and
- a study of all older people in New Zealand found that chronic anticholinergic or sedating medicine use increases the risk of a fall requiring hospitalisation by 56%, and increases visits to GPs. A similar large study in Australia found that taking two anticholinergics essentially doubles the risk of hospitalisation with acute delirium, and taking three anticholinergics approximately quadruples the odds of this same outcome.
Impressions of anticholinergic scales
I started reading more about this topic in 2012. It soon became clear that – confusingly – there existed at least six scales listing anticholinergic burdens of medications, published mostly from 2006 to 2011.
Nevertheless, memorable recollections were that some disparate drugs – ones I’d never have considered – apparently possess anticholinergic activity, and that this is something which may be bad for patients.
I vividly remember peering at the scales and thinking, “gosh, I had no idea warfarin, frusemide, ranitidine and tramadol may be anticholinergic”. At the time, I felt quite enthused at acquiring this seemingly niche knowledge, and recall hearing senior doctors say things such as “you know, olanzapine is the most anticholinergic of the antipsychotics” and “tramadol is bad for older people, it’s very anticholinergic”.
Australian data in older women from 2015 then added further intrigue, suggesting that in those patients for whom the overall anticholinergic burden of their drug regimen was high, the most common causal pattern was the accumulation of numerous “score 1” drugs (ie, many tiny anticholinergic burdens adding together to “perhaps” produce something more serious).
For example – though dependent on the published scale used – a patient may have a high total anticholinergic burden of 6 if they are on a cocktail of citalopram, atenolol, digoxin, frusemide, isosorbide and loperamide (each scoring 1 on certain scales). Each drug appears innocent on the surface, seemingly having no association with acetylcholine in the minds of doctors (at least not in mine). Nevertheless, this sum (of 6) is equivalent to the clout of taking two heavyweight anticholinergics, such as oxybutynin and doxepin (3 + 3).
Articles sometimes suggest that anticholinergic drugs are primarily only problematic in older people when they can cross the blood–brain barrier, thus linking to central neurocognitive side effects – simply because such drugs can enter the brain. However, I would argue that this need not be too heavily a focus, for two reasons:
- first, peripheral blocking of cholinergic synapses outside the central nervous system – namely in the parasympathetic system – can still be troubling; consider the fact that anticholinergics in this regard can cause tachycardia (and prolong QT interval), risking acute cardiac events; they can trigger long term catheterisation due to urinary retention, if not addressed; they could increase falls risk from patients being “blinded by the light” owing to pupillary dilation; and
- second, there is no easy way of measuring how well a drug crosses the blood–brain barrier, a property which is complex and multifaceted – and therefore much energy can be expended on this line-of-enquiry, with limited conclusiveness.
What is the overall message?
The take-home message from the concept of the anticholinergic burden is therefore to think laterally and beware hidden drug toxicity. The concept prods us to consider whether we’re inadvertently harming patients by the summative use of drugs possessing test tube or clinical anticholinergic tendencies. It also poses a call-to-action: given poor outcome data, shouldn’t we rigorously teach trainees to study the lists of anticholinergic medications, and commit to the potential deprescription of as many agents as possible raising the anticholinergic burden?
And so here we are:
- we have a relatively simple but solemn idea, published widely – that drugs can be quantifiably assessed for anticholinergic toxicity; and
- all we need to do is consult the relevant scales or tables to see where potentially “dodgy” medications may lie in the mix.
That’s the theory at least. As it has dawned on me (embarrassingly slowly) over 20 years, just because a concept seems watertight and authoritative doesn’t make it necessarily user-friendly or even employable at the bedside. In part 2, I will share some thoughts on the reality of trying to use anticholinergic burden scales in real life. Like an inebriated karaoke rendition of a rock anthem, it isn’t always pretty!
Dr Toby Commerford is a consultant geriatrician at Royal Adelaide Hospital, is course coordinator for geriatrics at the University of Adelaide’s Rural School, and practises remote and rural outreaches to Port Augusta and Murray Mallee. He is also the lead singer in a rock band.
The statements or opinions expressed in this article reflect the views of the authors and do not represent the official policy of the AMA, the MJA or InSight+ unless so stated.

 more_vert
more_vert
A very interesting and well argued presentation. When will the follow ups be available
Ben Goldberg
How does one determine the damage caused by ling term use of olanzapine in z person with intellectual disability prescribed off label since 1992, at a high dose, 20mg per day for over 10 years? Would this possibly cause problems with the myelin sheath I wonder
“Yay Anonymous” gave me a laugh and Toby dropped the pearl (at least, for a simple rural GP with minimal access to brainy physicians) late in the article- the example of the potentially cumulative score of 6 — well written all round!
You’ve taught me something, thank you.
You have the most beautiful way of writing. I didn’t really know what you were talking about, but it was like eating Jelly.
Yay
Interesting article highlighting that good clinical decisions in geriatric medicine require personalised care planning after a comprehensive multidisciplinary assessment of each patient. Therefore, no single scale or assessment on its own is going to be a ‘magic bullet’. We do not see many patients with a single organ problem with a single aetiology. However, a holistic approach does not detract from the importance of individual issues. The safety of prescribed medications and awareness of potential harm from important classes of drugs such as anticholinergics, is nonetheless inportant. Discussion, education, research, and its clinical translation into this area will help us find the right tools to practice safer medicine.
Thanks for your interesting article. The problem is not the concept of anticholinergic burden but rather ‘our’ increasing tendency to use scales to make easier or or speed up clinical assessment, or more likely replace clinical judgement.
Inventing and researching new scales has become a refuge for (dare I say) clinicians who don’t want to or are (dare I say) unable rely on clinical expertise, judgement or their experience in diagnosis – that is the old style finesse of good medicine.
Or they design new scales for the use of others, perhaps less able than themselves (?) to feed the pursuit of time saving, short cuts and, again let me dare, churn. In aged care/geriatrics, the home of scalabilizing medicine, the best and at the same time worst example of this use of scales is the MMSE invented by a well meaning (no doubt) team of psychiatrists the use of which over the years has evolved far and above what was first envisioned as a screening test.
The last time I mentioned a MMSE result to an experienced neuropsychologist their scornful look summarised what I, and many others, have recognised as a fine example how badly an otherwise useful tool in the wrong hands can lead to bad medicine.
There is some irony that those who have copyrighted this tool may one day bear the brunt of class actions against them to atone for the damage it has wrought over the years as a cruel and inaccurate tool – cruel for the tragic carers who have been told that their beloved one doesn’t have cognitive problems when the history and clinical (there it is again) findings point directly to the diagnosis.
It is true that we needed a tool to increase awareness of the nature and extent of the problem of dementia – but for this. What problems it has wrought – the degree to which the test has been misused and misunderstood has resulted in countless disputes, and also considerable cost to all (I have years of medicolegal disputation experience that attests to the scale (sorry) of this problem).
I could go on with other examples of the misuse of scales that have tried to replace time, effort, judgement and clinical experience but I will leave that for next time. In the meantime carry on and treat scales as they were meant – descale!
Excellent review of a very practical issue. The scales shown and the figures quoted are probably relevant in selected populations. However caution is needed as stressed by the author in applying these to individual patients with multiple variables, which cannot be estimated precisely in real life.
These scales are probably useful information, but not as absolute guidelines.
In real life when all variables are not precisely estimated it would be prudent to use a cautious trial of withdrawal of suspect agents.
I am a 70yo retired radiologist.
I experienced several episodes of irregular bradycardia, often occurring while swimming.
I believe that Escitalopram was a major contributing factor and also Magnesium which I was taking prior to swimming to prevent cramps. My problem has almost disappeared on ceasing these medications.
Could this be ‘anticholinergic burden’?
I also take Tramadol for backache but usually not prior to swimming.
Also Colchicine 0.5mg daily which I believe has been very helpful for my chronic shoulder tendinopathy/bursitis.
Your article is certainly ‘food for thought’. Thank you.
Good food for thought, Toby. Our specialty needs sceptics and iconoclasts to remind us that dogma of whatever origin needs to be confronted in the interests of our patients.