A new study suggests rough surfaces inspired by the bacteria-killing spikes on insect wings may effectively combat drug-resistant superbugs, including fungi, particularly in people with implants.
Bacterial, fungal and viral infections are an ever-present threat to our wellbeing. Almost 80 years ago, Alexander Fleming predicted that microbes could develop antibiotic resistance and, as predicted, microbes are now rapidly developing resistance to antibiotics. Multidrug-resistant bacterial and fungal infections pose a significant threat to patients undergoing implant surgery (here), as traditional antibiotic treatments are no longer effective in combating resistant pathogens (here).
Our team has developed an innovative solution to preventing antibiotic-resistant implant-associated infections (here). Originating from our investigations of natural self-cleaning surfaces, we discovered bacteria were killed on the wings of the cicada, dragonflies and damselflies (here). The tiny nanoscale spikes on the surface of cicada and dragonfly wings mediate the physical killing of microorganisms, which stretch and rupture when they attach to the wing topography (here).
Our team has developed various mathematical models (here) of the interactions of bacterial cells and nanostructured surfaces, showing how microbes are susceptible to the spikes. This is demonstrated in this video.
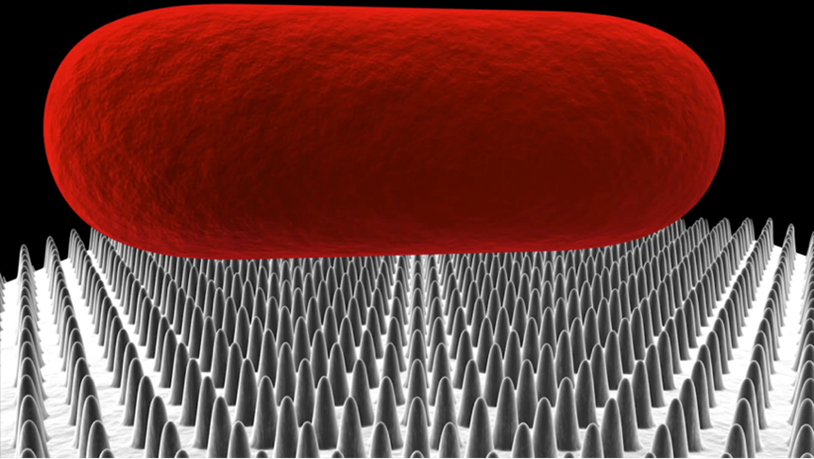
By incorporating these surfaces onto implant materials, we can create a surface that is hostile to multidrug resistant bacteria (here) and fungi (here), effectively preventing their attachment to the implant surface and eradicating those pathogens that do attach in a chemical-free way.
Motivation
Our motivation in this work was to tackle the challenge of Candida infections. Pathogenic yeasts are particularly resilient pathogens and account for an increasing percentage of implant-associated infections.
Candida spp can be highly antibiotic-resistant and readily form biofilms on medical and dental devices (here). Indwelling medical devices such as prosthetics, intravascular catheters, and ventricular assist devices (VADs) are commonly colonised with Candida spp (here). Adherent Candida within the biofilm detaches and can cause systemic fungemia (candidemia). Resultant infections are of significant clinical concern due to their severity and high fatality rate (here). Clinically, unless a swift diagnosis to treat a Candida infection is given in the first 24 hours, then there is a significant increase in likelihood of mortality (here and here).
In our recent work, we explored the potential of micro-structured titanium surfaces to eradicate multidrug resistant Candida species. We sought to understand how these surfaces could deter fungal biofilm formation for an extended period.
Findings
In this study, we etched titanium using a process called reactive ion etching to generate 5-micron tall pillars on the surface. We investigated the antifungal properties of the etched titanium surfaces against two Candida species, including multidrug resistant Candida auris.
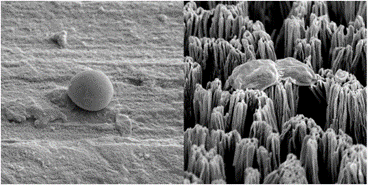
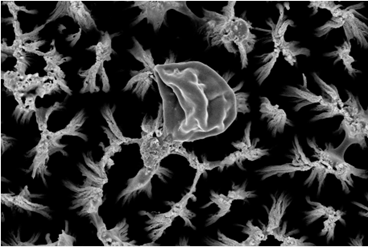
We found that the micro-pillars exhibited both fungistatic and fungicidal behaviour, preventing biofilm formation by consistently killing Candida cells over the course of one week. In addition, the surfaces injured cells, ensuring they could not form a biofilm or be recovered once introduced to a non-stress environment. After interaction with the titanium micro-pillars, injured Candida cells expressed elevated levels of protein (caspase, metacaspase and gasdermin D) involved in cell cycle arrest and triggering apoptosis. Yeast cell apoptosis is a manner of programmed cell death that is associated with the eradication of damaged cells. Programmed cell death was triggered because of physical damage to their protective cell wall. An increase in the number of identified proteins involved in intracellular protein transport and stress response indicated that the cells were subjected to physical stress upon contact with the micro-pillars. Furthermore, we determined that the injury to the Candida cell wall was the most likely cause of cell death as the production of proteins involved in the rebuilding of the cell membrane was upregulated.
Candida cell injury on micro-pillar surfaces was further associated with the downregulation of proteins involved in the development of the biofilm-forming phenotypes of yeast cells, confirming that the yeast cells were unable to form a biofilm.
Implications for implant patients and doctors
The outcomes of our research demonstrate that micro-pillar surfaces have remarkable antifungal properties against Candida species, including multidrug resistant strains.
The mechanisms of antifungal action involve physical rupturing by the micropillar structures and programmed cell death, specifically apoptosis, caused by physical damage to their protective cell wall and leading to unavoidable cell death.
Significantly, our research provides insights into the potential of modifying titanium implant surfaces to prevent biofilm formation and combat Candida infections.
By incorporating micro-nanostructured patterns onto implant material surfaces, it may be possible to significantly reduce the risk of Candida infections in implant patients and the further emergence of antibiotic resistance. These surfaces not only eliminate multidrug resistant Candida species but also prevent their attachment and subsequent biofilm formation, which is a common challenge in implant-related infections.
Our prior research has also demonstrated that micro-structuring of material surfaces enhances tissue integration by encouraging the attachment and proliferation of human cells, including stem cells and osteoblasts, and directs the osteogenesis of stem cells in the absence of growth factors (here). By addressing the key aspects of infection prevention and enhanced tissue integration, the longevity of implants can be enhanced, and the incidence of infection-related complications can be reduced. This has the potential to greatly improve patient outcomes.
For doctors and health care professionals, the application of antimicrobial surface structures to implantable materials represents a major advancement in the prevention of Candida infections in implant patients.
Conclusions
Our findings have significant implications for the development of biofilm-resistant surfaces and the potential to revolutionise the design and manufacture of implantable materials. Candida infections can be particularly challenging to treat, especially when they are multidrug resistant. Traditional antifungal approaches may not be effective in these cases, making alternative approaches crucial.
The incorporation of micro-structured surfaces on implantable biomaterials can reduce the stress of infection risk on patient and doctor care and improve patient outcomes after surgery.
Surface structures like micro-pillar patterns have the potential to transform the field of implantology, offering hope and a better quality of life for individuals at risk of Candida infections.
Acknowledgements
The authors acknowledge support from the Australian Research Council (ARC) Industrial Transformation Training Centre (ITTC) on Surface Engineering for Advanced Materials (SEAM) via award IC180100005; the Microscopy and Microanalysis Facility (RMMF) at RMIT University and the Melbourne Centre for Nanofabrication (MCN) in the Victorian Node of the Australian National Fabrication Facility (ANFF).
Elena Ivanova is a Distinguished Professor and founder of the Nanobiotechnology Laboratory at RMIT University, Melbourne.
Denver Linklater is a Postdoctoral Research Fellow at the University of Melbourne, Melbourne.
Vladimir Baulin is a Distinguished Researcher at Universitat Rovira I Virgili, Tarragona, Spain.
Saulius Joudkazis is a Professor and Founder and Director of the Nanofabrication Laboratory at Swinburne University of Technology, Melbourne.
The statements or opinions expressed in this article reflect the views of the authors and do not necessarily represent the official policy of the AMA, the MJA or InSight+ unless so stated.
Subscribe to the free InSight+ weekly newsletter here. It is available to all readers, not just registered medical practitioners.
If you would like to submit an article for consideration, send a Word version to mjainsight-editor@ampco.com.au.

 more_vert
more_vert