Targeted delivery of therapeutics will help combat the unfavourable biodistribution and low tumour cell penetration issues, providing an avenue that can have a significant impact on improving the quality of life for patients with ovarian cancer
OVARIAN cancer is a significant health issue with a lasting impact on the whole community. It is the most deadly of all gynaecological cancers, and the sixth most reported cancer and the fifth leading cause of cancer-related deaths in women worldwide and in Australia respectively (here and here). In Australia, over 1000 women died of ovarian cancer in 2021.
Epithelial ovarian cancer is a major cause of cancer morbidity and mortality in women. It is usually asymptomatic in early stages and, thus, is often diagnosed at an advanced stage.
Despite advances in surgery, chemotherapy, and radiotherapy over the past few decades, epidemiological studies indicate that these advances have had only a marginal impact on the course of ovarian cancer (here and here).
Current therapy is inadequate as most ovarian cancer cases present at an advanced clinical stage. The majority of patients are diagnosed at an advanced stage (eg, III or IV, when the cancer has spread or grown into nearby organs in or outside the pelvis) and have an extremely poor prognosis (5 years survival is ~27% for stage III, and 13% for stage IV). The few patients who present with properly staged, well differentiated International Federation of Gynecology and Obstetrics (FIGO) Stage I disease (ie, the cancer is only in the ovary [or ovaries] or fallopian tubes) have much better rates of survival (> 90%) (here and here).
The development of earlier and/or more effective detection tests, especially in high risk women to develop ovrian cancer, is undoubtedly the number one priority for long term reduction of the mortality due to ovarian cancer.
What is the solution?
In Australia, an estimated 1720 new cases of ovarian cancer were diagnosed in 2021, and 1042 deaths were reported.
There has been little improvement in survival rates over the past 20 years. A major contributing factor to the high mortality rate is the lack of clinically useful biomarkers for earlier detection of ovarian cancer. There is thus an urgent need for non-invasive and specific biomarkers to identify patients who have early stages of ovarian cancer.
Currently, only 29% of women diagnosed with advanced-stage ovarian cancer will survive beyond 5 years. But with an early detection test, that would skyrocket to over 90%
— Lucinda Nolan, CEO Ovarian Cancer Research Foundation
Although no ovarian cancer test has been developed that is suitable for community-based screening, evidence supports the hypothesis that certain epithelial ovarian cancers might be detectable up to 2 years before their clinical presentation (here and here). Should it become possible to screen for FIGO Stage IA disease (ie, the cancer is in one ovary and the tumor is confined to the inside of the ovary, with a 5-year survival rate of > 90%) with an accuracy of 80%, an overall ovarian cancer death reduction of the order of 50% could be achieved.
What is the challenge?
The development of earlier detection tests for ovarian cancer is challenging due to clinically ambiguous symptoms. This means patients often present to care with advanced stage disease, rather than during early stages.
Furthermore, due to the incidence rate of ovarian cancer being around six in 100 000 women (age-standardised incidence worldwide and Australia) even if a screening test has a specificity (ie, true negative rate) of 95%, this would still result in an unacceptably high false positive rate to be clinically useful due to the unnecessary laparotomies to diagnose ovarian cancer.
It is becoming clear that single biomarker approaches may never be capable of delivering a positive predictive value (ie, proportion of women with ovarian cancer who tested positive for the disease) in the range required for population-based ovarian cancer screening. Consequently, it is imperative that additional strategies be brought to bear on this important and challenging clinical problem.
Contemporary approaches have focused on plasma or serum biomarkers (including CA125 for confirmation of diagnosis and monitoring treatment response), and imaging techniques. Although a proportion of asymptomatic ovarian cancers can be identified by measurement of plasma CA125, by real-time ultrasonography and by colour-flow Doppler, these screening modalities, even when applied in combination, have an unacceptably low positive predictive value, and to date, no effective population-based screening strategy for ovarian cancer exists (here and here).
Fingerprinting cells: a new methodology
Recent studies have established that extracellular vesicles – and, in particular, exosomes – mediate communication between normal and cancerous cells (here, here, here, here, here, here and here).
Exosomes are a type of small vesicle that are released from a wide range of cells, including healthy and cancer cells. They are packaged with specific signalling molecules that vary depending on the cell type that released the exosome.
Once released, exosomes are capable of regulating target cell behaviour, inducing a favourable environment for tumour cells and thus contributing to tumour growth and spread, as well as influencing cell function in many areas of the body.
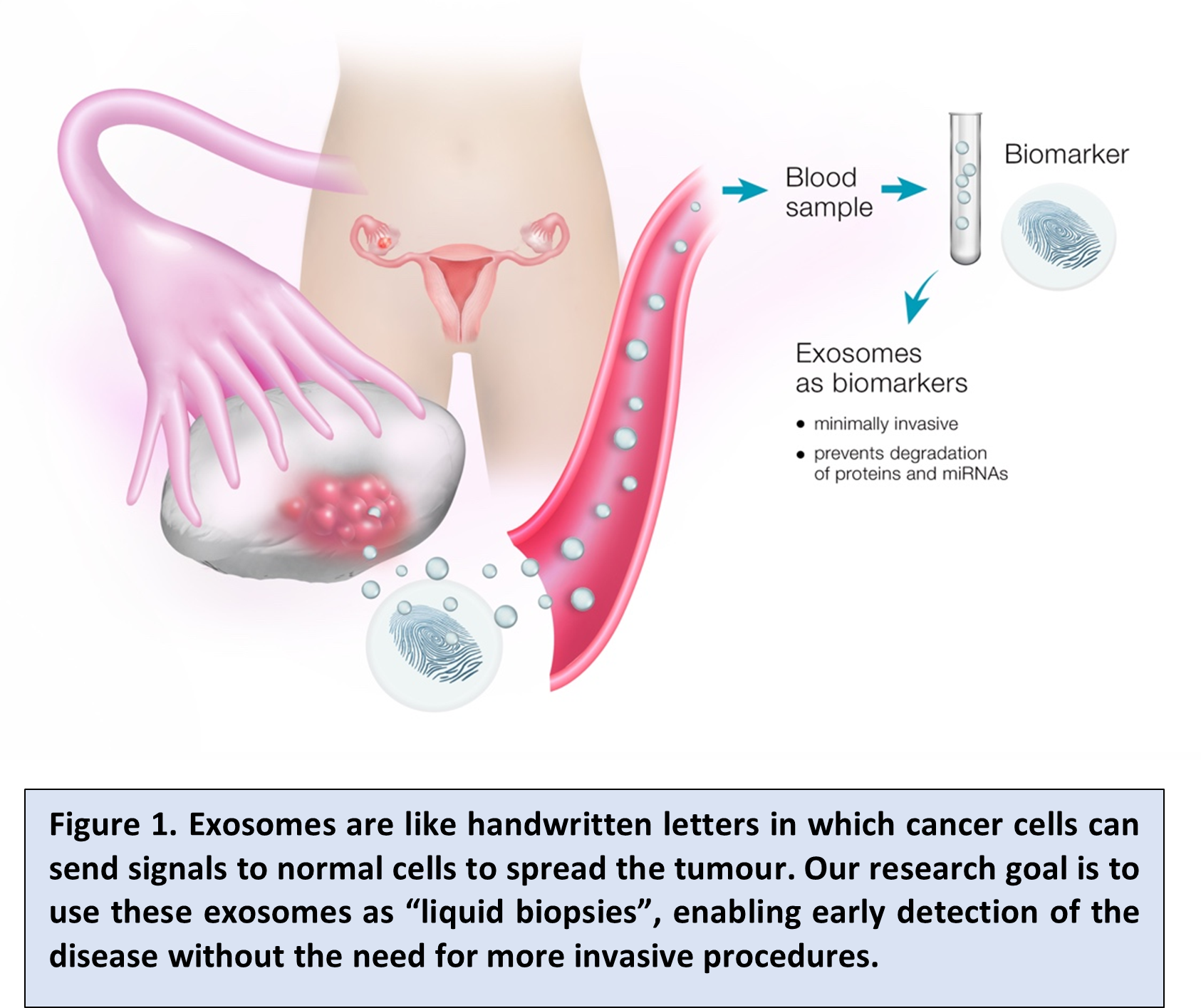
Recently, we proposed that exosomes represent a “fingerprint” of their cell of origin and reflect the processes happening within that cell (Figure 1). Therefore, these exosomes may potentially identify otherwise “hidden” tumour cells and provide us with an insight into the cells, thus giving information on tumour cell presence and/or behaviour, likely aiding early diagnosis.
OCRF-7: a promising building block for a diagnostic test
Recently, our team at the University of Queensland completed a retrospective case–control discovery project in around 500 women funded by the Ovarian Cancer Research Foundation (unpublished results).
This project identified an informative exosomal signature in the blood of women with early-stage high grade serous ovarian cancer (responsible for most of the disease mortality). The biomarkers that were identified are associated with a recently identified extracellular signalling pathway used by cancer cells to increase cancer spread.
Based on this signature, a test was developed (OCRF-7), using machine learning approaches, and clinically validated in an independent sample set. The classification efficiency of the test for early stage cancer was > 90% in symptomatic patients. This suggests that it could be ideal as a first line test for population screening.
To explore this, we will assess the performance of the test in a case–control sample set from the largest ovarian cancer screening trial: the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) (here, here and here). The results will pave the way for evaluating the test in a prospective screening trial and, in due course, clinical implementation.
While surgery and chemotherapy have afforded some progress in improving survival rates for women with ovarian cancer, the rates have not changed significantly over the past 20 years, both worldwide and in Australia (eg, in 1998 the mortality rate was 7.3/100 000 v 6.5/100 000 in 2018). The major factors limiting progress are a lack of clinically useful early detection markers for disease and the unavailability of a useful marker for assessing response to chemotherapy.
Furthermore, reduced penetration of therapeutic agents into tumour cells combined with unfavourable biodistribution are also limiting factors. Therefore, it is essential that a non-invasive, specific marker be developed to identify patients at risk of disease recurrence, and/or resistance to therapy, thus allowing implementation of personalised treatments. In addition, targeted delivery of therapeutics will help combat the unfavourable biodistribution and low tumour cell penetration issues, providing an avenue that can have a significant impact on improving the quality of life for patients with ovarian cancer.
Associate Professor Carlos Salomon is Head of the Exosome Biology Laboratory at the University of Queensland (UQ) Centre for Clinical Research. He is a National Health and Medical Research Council Investigator Fellow, and is supported by the Ovarian Cancer Research Foundation (OCRF), Lion Medical Resarch Foundation (LMRF), the Donald and Joan Wilson Foundation, and the Medical Research Future Fund (MRFF). INOVIQ (ASX:IIQ) has the exclusive option to license rights to the development and commercialisation of UQ’s exosome-based early detection test for ovarian cancer (OCRF-7).
The statements or opinions expressed in this article reflect the views of the authors and do not necessarily represent the official policy of the AMA, the MJA or InSight+ unless so stated.
Subscribe to the free InSight+ weekly newsletter here. It is available to all readers, not just registered medical practitioners.
If you would like to submit an article for consideration, send a Word version to mjainsight-editor@ampco.com.au.

 more_vert
more_vert
It was extremely stressful to learn that I may have ovarian cancer and a frightful wait to have it surgically removed and tested to check if it was malignant or benign. Thankfully it was benign and I hope it does not reappear in the healthy ovary. The statistics for survival are very alarming and the work you are doing is so important for early detection. Thank you!
More GPS need to be educated on TMS