PATIENTS with spasticity due to stroke and other neurological illnesses describe botulinum toxin as a wonder drug that enables them to regain control of their hands and feet. So why is the treatment not more widely available in Australia?
In a Perspective published online in the MJA, Dr Anupam Datta Gupta, a rehabilitation specialist at Adelaide’s Queen Elizabeth Hospital, and public health academic Dr David Wilson called for botulinum toxin to be publicly funded to treat upper and lower limb spasticity and dystonia, which can stem from a range of neurological illnesses including stroke and Parkinson’s disease.
“Spasticity can be moderated safely and effectively in both upper and lower limbs with botulinum toxin and the input of a skilled multidisciplinary team,” they wrote. “Individuals with spasticity are already disadvantaged by their condition; they should not be further disadvantaged by ignoring effective treatment options.”
The Pharmaceutical Benefits Scheme (PBS) currently only funds botulinum toxin injections for stroke patients with upper limb spasticity – but not lower limb spasticity – and for selected patients with cerebral palsy.
Dr Gupta and Dr Wilson argued that the limited PBS criteria failed to take into account the latest evidence.
“Botulinum toxin has emerged as the drug of choice for focal spasticity and dystonia because treatment can be targeted and a single treatment can last up to 4 months,” they wrote.
“Use of the toxin also creates a window of opportunity within which intensive physiotherapy and other therapies can be conducted.”
The US Food and Drug Administration recently approved botulinum toxin injections for lower-limb spasticity in patients who had a stroke. The approval followed positive results from an industry-sponsored double–blind, randomised, placebo controlled trial in 468 patients across multiple sites, which showed improvements in muscle tone and Clinical Global Impression in the treatment group.
The South Australian Government currently funds botulinum toxin injections to treat spasticity for some indications not covered by the PBS. The injections must be given in a multidisciplinary setting with regular patient outcomes assessments.
Dr Gupta, who estimated he has treated more than 150 patients under the provisions, told MJA InSight: “It takes about 4–7 days for the injections to take effect, and together with physiotherapy and exercises, we see some patients regaining their ability to do things like walk and drive and better perform their hygiene and bladder function”.
Mandy Bosson has been receiving 6-monthly botulinum toxin injections and physiotherapy to treat spasticity of her upper and lower limbs resulting from a stroke 6 years ago.
“It helps me to do normal things like drive,” Ms Bosson told MJA InSight. “I find it harder to walk when the [botulinum toxin] is wearing off. My leg tends to go inwards as a result of the spasticity and I am at risk of tripping over.
“I don’t know what I would have done without the ongoing injections – it makes such a difference.”
Richard Krantz, who has Parkinson’s disease, has been receiving botulinum toxin injections to treat foot dystonia.
“Parkinson’s makes my toes curl over one another. It’s very painful – especially in bed at night –and it makes it hard to walk,” he said. “The injections help me dramatically. People should have access to this when they need it.”
However, Professor Kerr Graham, Director of the Hugh Williamson Gait Analysis Laboratory at the Royal Children’s Hospital in Melbourne, has called for caution about expanding the use of botulinum toxin.
“No doubt there are some patients in any cohort who have a dramatic response, but they may not be typical of the whole group,” he told MJA InSight.
Professor Graham has pioneered the use of botulinum toxin to treat spasticity in children with cerebral palsy for the past 30 years. However, he said recent animal studies raised concerns about the safety and reversibility of the treatment, especially when used frequently and in the relatively large doses needed to treat limb spasticity.
“Botulinum toxin is not what we thought it was,” Professor Graham said. “We used to think that muscles recovered completely 3–6 months after the injection, but the animal studies show that muscles do not recover fully, especially if injected frequently.”
“In simple terms, some of the muscle gets replaced by fatty tissue in the short term and, in the longer term, by fibrous tissue,” he said.
“In children with cerebral palsy, I believe that we need to decrease our use of botulinum toxins because we have used them too often and for too long,” he said. “I think we need more randomised controlled trials before considering the extension of the use of botulinum toxin in many neurological conditions.”
Dr Gupta responded positively to Professor Graham’s concerns.
“I’m in full agreement that botulinum toxin injections should be used discriminately. The doctor needs to do a careful assessment to ensure the patient has focal spasticity – not contracture – otherwise the treatment won’t work, and there must be careful follow-up,” he said.
“With careful patient selection and follow-up, we aren’t seeing any significant side effects.”
The Rehabilitation Medicine Society of Australia and New Zealand (RMSANZ) in 2017 published a position statement saying that current funding restrictions for botulinum toxin “are not reflective of modern best practice”.
The society wants the PBS to cover the treatment of focal spasticity of the upper and lower limbs, regardless of aetiology.
Dr Gupta said that the treatment costs up to around $2000 in adults, although there is evidence it is cost-effective.
A spokeswoman for the federal Department of Health said that the Pharmaceutical Benefits Advisory Committee (PBAC) had recently met with representatives from RMSANZ to discuss the requirements for a submission to broaden the PBS subsidy of botulinum toxin to include spasticity and dystonia in adults.
“The PBAC would welcome a submission at any time,” she said. “The PBAC last considered a submission for botulinum toxin for lower limb spasticity in July 2008. That submission was rejected because of uncertain clinical benefit and the resulting high and uncertain cost-effectiveness.”
To find a doctor, or a job, to use GP Desktop and Doctors Health, book and track your CPD, and buy textbooks and guidelines, visit doctorportal.

 more_vert
more_vert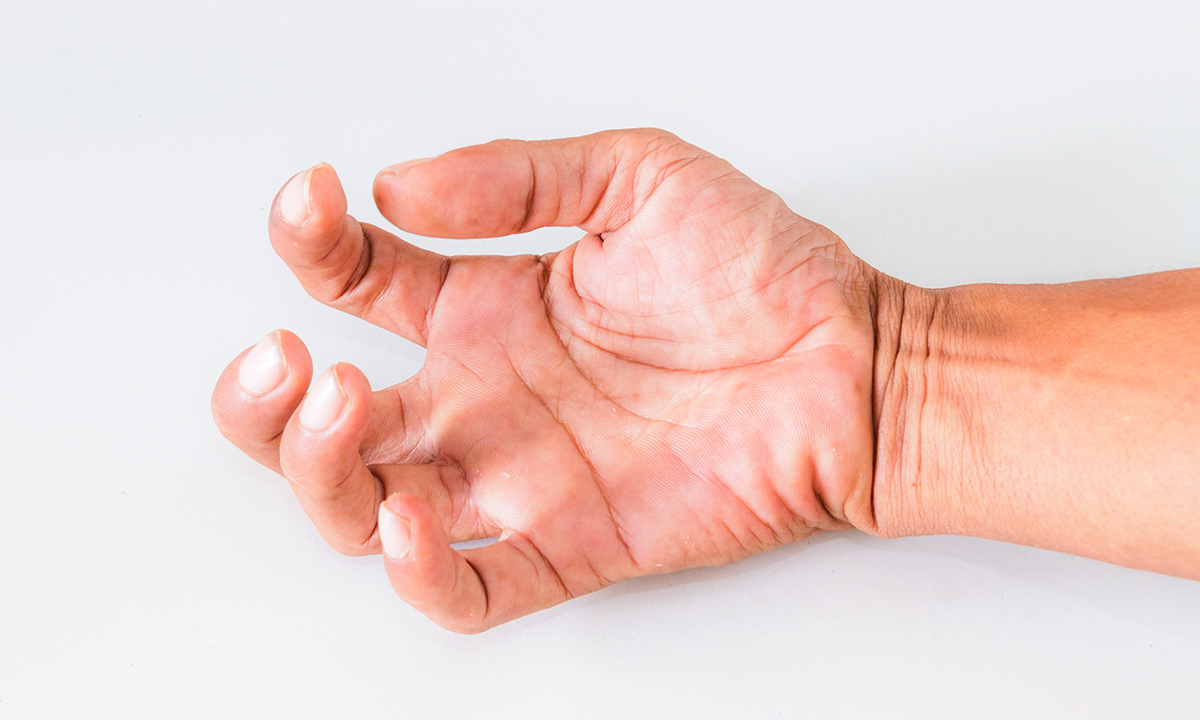
I have cervical dystonia and have applied botox for over 8 years. It seems to me that we need a better specific device to apply the botox. It is really difficult for the doctor , even mine who has been my doctor all this time, to be sure If the muscles He is apllying the botox are the right ones…Sometimes the same muscles that showed spasmodic movements once…the next time i apllly the botox, they seems to have only contracture and not so strong spasmodic movements.
Are there any researches About It? New devices that could help in clinic?
Best regards,
Maitê Jonsson.