STRONG evidence suggests that transcranial magnetic stimulation (TMS) is a safe and effective treatment for drug-resistant depression, but the lack of a Medicare item number is keeping it inaccessible to most patients.
In a Perspective published by the MJA, Professor Saxby Pridmore, professor of Psychiatry at the University of Tasmania and a TMS researcher at St Helen’s Hospital in Hobart, wrote that the “evidence proves [TMS] to be safe, potent and cost-effective in treating resistant major depressive disorder (MDD), and comprehensive psychiatric services should provide both electroconvulsive therapy (ECT) and TMS”.
TMS involves creating an electromagnetic field, using an electromagnet placed on the hair or scalp to generate minor electrical activity in a localised region of the cortex. Unlike ECT, TMS does not involve anaesthetics, seizures or memory disturbance, and may be more widely acceptable.
Professor Pridmore, speaking in an exclusive podcast with MJA InSight, said that TMS was “beautiful science”.
“If you put a paper clip on top of a table and a magnet underneath, you can move the paper clip with great precision,” he said. “[In TMS] we use an electromagnet … magnetic lines of force pass straight through non-conductors [such as the human skull].
“What happens with TMS is you get magnetic lines of force that go through the skull, and if you stop and start the magnetic field, that creates a little tiny electrical field, so it goes electricity–magnetic–electricity. Being able to put the little magnetic lines through the skull where you want them, and create a little electrical field inside, you can be very precise,” Professor Pridmore said.
“Isn’t that beautiful?”
ECT in comparison was “a bit of a blunderbuss”, he said.
In his MJA Perspective Professor Pridmore laid out the evidence for TMS, writing that “the safety and therapeutic benefits of TMS in the treatment of MDD that has not responded to medication was first shown in 1995”.
“Subsequently, there have been at least 59 sham controlled trials, with most of them finding beneficial effects. In addition, there have been 30 systematic reviews and meta-analyses on the effects of TMS, and also naturalistic studies that have shown the safety, tolerability and effectiveness of TMS in the treatment of medication-resistant MDD in the real-world clinic.”
TMS, he told MJA InSight, was “just about as effective as ECT”.
Treatment of MDD with TMS usually involves a course of 20–30 treatments delivered over 4–6 weeks.
Relapse after treatment with TMS, like with ECT, was a possibility, said Professor Pridmore. Top-up treatments were then an effective option.
“You may relapse. That doesn’t mean that the treatment is weak. What it means is that the disorder is strong,” Professor Pridmore said.
“We are treating people five times in 3 days and we do that about every 6 weeks. The thing is not to wait until the person has had a huge relapse. Get in after about 4 or 6 weeks. If they are saying ‘look, I’m starting to lose it,’ then we give them a ‘top-up’ treatment. We call it early-relapse TMS.”
However, the cost of TMS to the patient, given the lack of a Medicare item number, is prohibitive.
“Except for those patients who are able to access one of the few public TMS services, this treatment is available through private outpatient services and has a cost of $170–300 per treatment and a total cost of around $6000 per course, which, naturally, excludes most patients,” Professor Pridmore wrote in the MJA article.
“Moreover, another route to TMS treatment is available to some privately insured patients: some private hospitals meet the costs of TMS services for admitted patients through insurance company contributions to the cost of hospitalisation. However, such arrangements may lead to patients waiting for sufficient deterioration to justify hospitalisation, or seeking hospitalisation before it is necessary to gain treatment — neither of which is optimal.”
TMS has been up before the Medical Services Advisory Committee (MSAC) twice before. In 2007, it was rejected for a Medicare item number because, in part, “TMS was incorrectly believed to have less antidepressant potency than ECT”, according to Professor Pridmore. In 2014, it was refused mainly because of “questions about the cost effectiveness of TMS compared with other treatments, but recent overseas studies have found TMS to be more cost-effective than ECT”, he wrote.
Professor Pridmore talks in more detail about the cost effectiveness of TMS versus ECT in the MJA podcast.
A third application is currently before the MSAC.
“The treatment of MDD has enjoyed few forward leaps,” wrote Professor Pridmore. “ECT in the 1930s and the monoamine oxidase inhibitors (followed by the tricyclic antidepressants) in the mid-20th century are the most notable examples, and it is argued that TMS is potentially of this order of importance.
“TMS has been a field of study and of limited clinical service for over two decades … An item number for TMS would enable equitable availability and proper use of this important therapeutic advance.”
To find a doctor, or a job, to use GP Desktop and Doctors Health, book and track your CPD, and buy textbooks and guidelines, visit doctorportal.

 more_vert
more_vert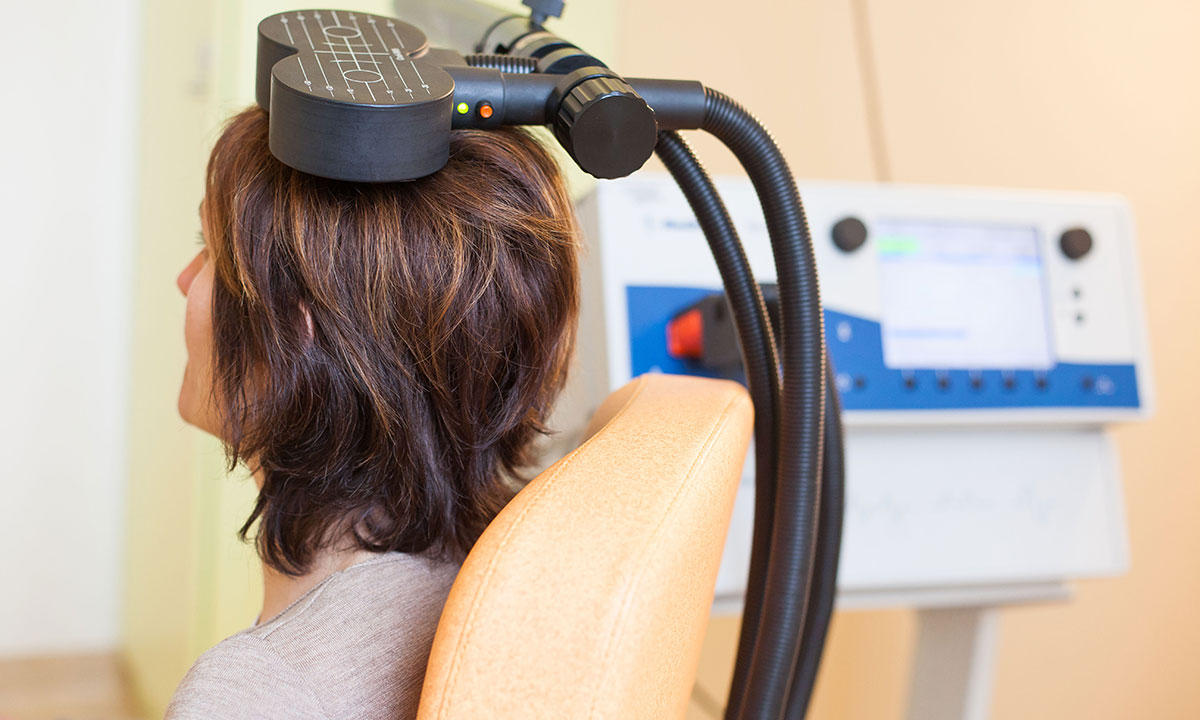
What was the outcome of the third application? I can find a record of it it in July 2018 but cannot find any outcomes