In 1984, dexamphetamine became available in Australia as a subsidised medicine for treating attention deficit hyperactivity disorder (ADHD). Immediate release and long-acting methylphenidate, atomoxetine and modafinil have since been added to the Pharmaceutical Benefits Scheme. Apart from atomoxetine (a noradrenaline re-uptake inhibitor), these medications are all psychostimulants. With the exception of dexamphetamine, the rates of prescribing of these medications continues to grow significantly in Australia and elsewhere.1,2 This can largely be attributed to the increasing acceptance of ADHD as a diagnosis.3,4 However, these trends in prescribing are occurring amid increasing concerns about the diversion and misuse of these drugs.5 Further, there is increasing public anxiety about prescription stimulant dependence and misuse, including warnings about illicit injection of methylphenidate (Ritalin) following two recent deaths in Tasmania.6 This is in the context of much wider concerns about the relatively high prevalence of methamphetamine dependence in Australia7 and the increased use of methamphetamine by existing drug users,8 which may be related to recent increases in the purity of illicit crystal methamphetamine.9 There is also growing unease about the overdiagnosis and overtreatment of ADHD, as well as about the increasing off-label use of these medicines to treat conditions for which the evidence base is weaker.10
The factors described here contribute to the increasing public availability of ADHD medications that can be diverted, misused and lead to overdoses. In this article we describe trends in intentional exposures to ADHD medications (overdoses and recreational use) reported to the New South Wales Poisons Information Centre (NSWPIC) over an 11-year period.
Methods
Data sources
We conducted a retrospective study of calls to the NSWPIC from 1 January 2004 to 31 December 2014. The NSWPIC receives about 110 000 calls from the public and from health care professionals each year, accounting for around 50% of Australian PIC calls. Between 6 am and midnight, the NSWPIC receives calls from NSW, Tasmania and the Australian Capital Territory, and it also handles after-hours calls from across Australia for seven nights each fortnight.
The defined daily dose per 1000 population per day (DDD/1000/day) is a measure of the national use of a drug, and this information is published annually in Australia.11 We extracted the data for the medicines of interest during the study period (2004–2014).
Search strategy and inclusion criteria
We searched the NSWPIC database for “methylphenidate”, “dexamphetamine”, “modafinil” and “atomoxetine”. We also searched for “methamphetamine” to allow comparison of the number of calls about prescription medications with that of calls related to definite illicit amphetamine use. Since our focus was intentional exposures, we excluded exposures in children under the age of 10 years to remove paediatric ingestions of accidental or undetermined intent. We therefore included in our analysis only exposures coded as “intentional” in people aged 10 years or more. The co-ingestion of alcohol or illicit street drugs, such as ecstasy, marijuana or cocaine, was used as a proxy measure of illicit use. Characteristics, including age, sex, co-ingestants, route of exposure and symptom disposition, were extracted. The presence of symptoms at the time of the call was coded as “present” or “absent”.
Definitions
For the purposes of this study, misuse was defined as using the drug for a purpose other than that for which it was prescribed. This included taking the drug in excessive quantities or by non-oral routes, and the use of diverted medicines. Diversion was defined as the transfer of drugs from legal sources to an illicit channel or marketplace.
Statistical analysis
Statistical analyses were performed with the Joinpoint regression program, version 4.2.0.2 (Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute)12 and SPSS for Windows 22.0 (SPSS Inc). Continuous data were summarised as medians and interquartile ranges (IQRs). Time trends in the ratios of calls to all intentional poisoning calls (to adjust for annual call fluctuations) and of calls to DDD/1000/day (to adjust for changes in dispensing) were analysed with the Joinpoint program. This program tests for joinpoints (points where there is a significant change in trend); it provides measures of annual percentage change (APC) for each trend segment and an average annual percentage change (AAPC) for the entire study period. The program then tests whether these measures differ significantly from zero (α = 0.05). In addition to examining overall trends, analysis was stratified by age category (children, 10–14 years; adolescents, 15–19 years; adults, 20–75 years) and sex.
Ethics approval
Ethics approval was obtained from the Human Research Ethics Committee of the Sydney Children’s Hospitals Network (approval number, LNR-2011-04-06).
Results
During the 11-year study period, 1735 calls were received by the NSWPIC about intentional exposures to ADHD medications (dexamphetamine, 575 calls; methylphenidate, 1059; atomoxetine, 83; modafinil, 18). Intentional exposures constituted 42% of all exposure calls for these drugs. Characteristics of the subjects in this study are summarised in Box 1. Their median age was 17 years (IQR, 15–23 years). Only 4% of exposures were by injection and 1% by inhalation or other nasal ingestion; 95% were by oral ingestion. Injected use of methylphenidate increased during the study period (three cases in 2004, ten in 2014). At the time of the call to the NSWPIC, 60% of cases were symptomatic and 26% asymptomatic; symptom status was not recorded for 14% of calls. At least 93% of calls resulted in hospitalisation (the call originated from a hospital, or the subject was referred to hospital). Consultant toxicologists were involved in the management of 60 cases (3% of calls).
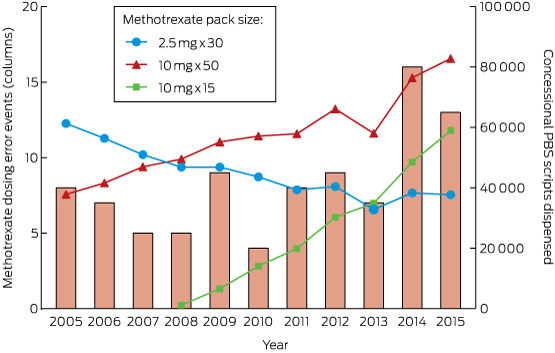
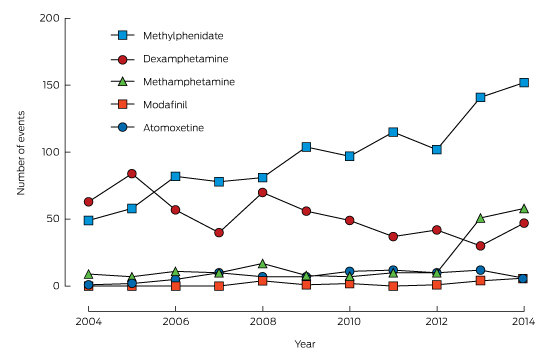
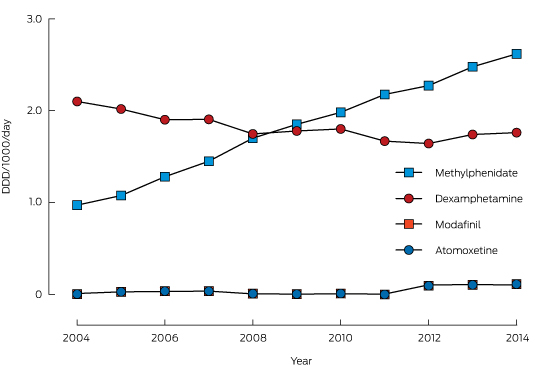
A summary of the unadjusted exposures over time is shown in Box 2. Most intentional exposures were to methylphenidate and dexamphetamine. There was a 210% increase in the annual number of exposures to methylphenidate during the study period, while dexamphetamine exposures declined by 25%. Modafinil and atomoxetine exposures were infrequent. Box 3 depicts the trends in dispensing of these medications (DDD/1000/day).
Methylphenidate exposures (expressed as a ratio of all calls about intentional exposures) increased significantly over the study period (Box 4; Appendix 1, A), with an AAPC of 9.8%. Dispensing of methylphenidate also increased significantly (Box 4; Appendix 1, B), with an AAPC of 10.8%; one joinpoint was identified, with the rate of increase slowing from 2008. When call numbers were adjusted for DDD/1000/day, there was no significant trend in the number of intentional calls about methylphenidate (Box 4), suggesting that trends in call numbers were associated with changes in the rate of medical dispensing.
Dexamphetamine exposures declined significantly from 2004 to 2014 (Box 4; Appendix 2, A), with an AAPC of −6.6%. Dexamphetamine dispensing (Appendix 2, B) decreased significantly between 2004 and 2012, with an APC of −2.7%, with no significant trend for 2012–2014 (Box 4). As with methylphenidate, there was no significant trend when the number of calls to the NSWPIC was adjusted for DDD/1000/day.
Results stratified by age and sex are shown in Box 5. The trends are similar to those in the unstratified data. The reduction in dexamphetamine exposures was only significant in adult women, adolescent men, and boys. The increase in the number of methylphenidate calls was significant for both sexes and for all age categories, with the exception of boys.
We also examined trends in illicit use, defined as use of the medication together with alcohol (146 calls) or any street drug (43 calls). Illicit use increased by 429% across the study period. Joinpoint analysis identified a significant increase in illicit use from 2004 to 2014 (Box 4; Appendix 3, A), with an AAPC of 13.8%. Further, there was also a significant increase in the number of reported exposures to methamphetamine (Box 4; Appendix 3, B), with an AAPC of 23.4%.
Atomoxetine and modafinil exposures and intravenous exposures were excluded from detailed joinpoint analysis because of the low numbers of calls about these drugs.
Discussion
The number of calls to the NSWPIC about ADHD medication exposures increased dramatically during the study period, driven mostly by calls about methylphenidate. While our joinpoint analysis showed a significant increase in the number of methylphenidate exposures and a significant decrease in that of dexamphetamine exposures, there were no significant trends after call numbers were adjusted for the dispensing rates for each medicine, indicating that call frequency had changed in line with prescribing rates. This finding was consistent with other PIC studies in which exposure trends were correlated with the sale or prescribing rate (and thus with the availability) of dexamphetamine and methylphenidate, but not of atomoxetine.5,13,14
This study was unable to capture whether the individual was prescribed the medicine involved in a call, or the reasons for an overdose. However, a Danish PIC study found that methylphenidate had been prescribed at the time of exposure in 65% of overdose cases; of these, attempted suicide (54%) and recreational use (40%) were the most frequent reasons for exposure.15 A further study, comparing exposures related to the non-medical use of atomoxetine and methylphenidate, found that recreational use was more frequent in methylphenidate (40%) than atomoxetine exposures (19%), with suicide attempt or emotional strain more common in atomoxetine (62%) than methylphenidate exposures (54%).13
The number of symptomatic patients in our study is consistent with the findings of other international studies. In the Danish PIC study, 323 patients (86%) were symptomatic; the symptoms presented were predominantly central nervous system/constitutional (altered psychomotor activity, mood symptoms, perceptual disorders; 81%) or cardiovascular (70%) in nature.15 A Swiss PIC study found a similar spectrum of toxicity for methylphenidate, with most people reporting mild to moderate symptoms.14 In our study, most calls resulted in hospitalisation, suggesting a high degree of overdose severity. A report from RADARS, an American prescription drug monitoring system incorporating PIC and diversion surveillance methods, found significant increases in the severity of overdoses associated with extended release amphetamine and methylphenidate.5
Our study found that the route of administration of these drugs was oral in most cases, but rates of intravenous administration were increasing. This is concerning because of the greater morbidity associated with intravenous use, and raises the question of whether those who inject these drugs are new or existing injecting drug users. The potential for harm associated with injection of these agents is reportedly comparable with that of amphetamines or cocaine.16 Other PIC studies have found similar trends in exposure routes, with oral ingestion being the most common, followed by injection (5–10%) and snorting (4–13%).14,15 In contrast, a study of regular ecstasy users found that up to 43% reported snorting the drug, but only a small number described injecting or smoking it.17
There has been a dramatic increase in the number of calls to the NSWPIC about incidents in which alcohol or illicit drugs were co-ingested with ADHD medicines, predominantly with methylphenidate, suggesting that misuse of this drug is increasing in Australia. In our study, this increase in suspected illicit use has been most marked since 2012, corresponding to a rise in the number of methamphetamine-related calls. This may be related to an increase in supply and demand for amphetamines for misuse, or with increasing toxicity among existing amphetamine users. Increases in methamphetamine purity were also seen around this time.8 Similar rates of co-ingestion were found by previous PIC studies.14,15 The United States National Survey on Drug Use and Health data for 2002–2009 indicated that 3.4% of those aged 12 years and over had used ADHD medications for non-medical purposes.18
Unfortunately, no equivalent national data are available for Australia. A recent systematic review of the misuse of prescription methylphenidate and amphetamine found that young adults (16–25 years), people being treated for ADHD, and known illicit substance users are at particular risk of misusing these medications. However, these studies were not population-wide, and may have been biased by focusing on specific populations. Studies of pharmaceutical stimulant misuse among US university and college students have yielded lifetime prevalence estimates of 7–17%, compared with a general population lifetime prevalence of 0.3–2.1%.19 In terms of current drug users, the results of a 2013 Australian survey of people who inject drugs found ADHD medication misuse to be uncommon.20 Conversely, half of a sample of regular ecstasy users reported illicit use of pharmaceutical stimulants at some point, and 30% reported using them in the past six months. Most of this misuse was of diverted rather than of prescribed medications.17 A US study found that two-thirds of those who used illicit drugs in addition to ADHD medications had begun using them before they had started an ADHD medication.18
Misuse has been described in people who are prescribed ADHD medications.19 In one study, as many as 14.3% of 545 respondents from an ADHD treatment clinic indicated that they had misused prescription stimulants at least once. Further, 39.1% of respondents also used non-prescription stimulants, particularly cocaine (by 62.2% of those who misused stimulants), methamphetamine (4.8%), or both cocaine and amphetamine (31.1%).21 However, another study found that people treated for ADHD in childhood do not appear to be at increased risk of illicit substance-related death, crime, or hospital visits later in life than children diagnosed with ADHD diagnosis who were not treated with stimulant medications.22
In studies examining reasons for misuse of ADHD medications, the most frequently reported were to improve attention, concentration and alertness, to improve study habits and academic performance, and to “get high”.19 However, the frequencies of these responses tend to reflect the populations most commonly studied, including college students and people with ADHD. A minority of people also report self-medication of undiagnosed ADHD symptoms.19 Of those who misuse these medications, the most common source is diversion from a friend, relative or dealer.20
According to reports by a group of regular ecstasy users, the median age of first use of ADHD medications by recent users was 18 years (range, 6–30 years) and the median amount taken in an average session was two tablets (range, 0.33–30 tablets).17 Other than this, little is known about the natural history or prevalence of ADHD medication misuse in Australia. The relatively young median age of first use found by this and other studies may be related to ease of access within peer networks.
The effectiveness and tolerability of atomoxetine are comparable with those of methylphenidate,23 but it is often prescribed only as a second-line drug, or for those perceived to be at risk of psychostimulant misuse.15,24 Atomoxetine exposures in our study appeared to result in less severe toxicity and to not be associated with illicit use, but the number of calls was too low to reach any conclusions. Significant toxicity associated with atomoxetine overdose has been described by other authors.25
Australian PIC data (together with data from other sources, such as hospital presentation data, police data, and wastewater analysis) are underutilised in detecting emerging trends in substance use. However, limitations of this retrospective study include the lack of outcome data, as Australian PICs do not routinely conduct follow-up calls. As specific symptoms were not coded, we were unable to construct a comprehensive symptom severity profile. Further, this study was limited to exposures in people aged 10 years or more, so that we are unable to comment on intentional poisonings in younger persons. Our data comprised a collection of all intentional exposures reported to the NSWPIC that does not allow further sub-categorisation into self-harm, recreational use, and other forms of misuse, so that co-ingestion of alcohol and illicit drugs were used as a marker of illicit intent. Alcohol is unlikely to be a specific marker, as it may also be taken with a self-harm overdose. A deeper understanding of these aspects of toxicity could be gained from examining poisoning cohorts, such as the Hunter Area Toxicology Service database.26 In addition, as PIC data collection relies on voluntary calls, our study is likely to have significantly underestimated the true frequency of the toxicity and misuse of ADHD medications. However, benchmarking to methamphetamine calls at least allows us to comment on trends. Our study predominantly reflects the NSW experience, with limited data from the rest of the country (resulting from the on-call system), so it may not be possible to generalise our findings to the rest of Australia.
Clinical implications
The incidence of poisoning exposures appears to be correlated with community prescribing rates. Misuse of these medications mostly involves diverted drugs, so that increasing prescribing of these medications is likely to increase their availability for diversion. There also appears to be an increased risk of misuse and overdose in people who are prescribed ADHD medications, but such misuse is often related to a past history of other drug misuse. In a similar vein to opioid management, care should be taken when initiating these medications to complete a full risk assessment for misuse, which includes taking an addiction history, and ensuring that they are used safely, appropriately and in an evidence-based manner, including considering non-medical or non-stimulant alternatives. Atomoxetine may be an alternative for those at risk of misuse. There may also be scope for regulatory measures and guidance. An example of this is the Stimulant Regulatory Guidelines developed in Western Australia in response to one of the highest global rates of stimulant use disorder.27
Box 1 –
Characteristics of 1735 intentional exposures to ADHD medication reported to the New South Wales Poisons Information Centre, 2004–2014
|
|
Dexamphetamine
|
Methylphenidate
|
Modafinil
|
Atomoxetine
|
Total
|
|
|
Number of reports (percentage of all exposures to ADHD medications)
|
575 (32%)
|
1059 (62%)
|
18 (1%)
|
83 (5%)
|
1735
|
|
Median age (interquartile range), years
|
19 (16–31)
|
16 (14–20)
|
30 (21–36)
|
16 (14–19)
|
17 (15–23)
|
|
Sex
|
|
|
|
|
|
|
Male
|
269 (47%)
|
503 (47%)
|
6 (33%)
|
42 (51%)
|
820 (47%)
|
|
Female
|
273 (47%)
|
499 (47%)
|
12 (67%)
|
32 (39%)
|
816 (47%)
|
|
Not recorded
|
33 (6%)
|
57 (5%)
|
0
|
9 (11%)
|
99 (6%)
|
|
Treatment
|
|
|
|
|
|
|
Hospitalised
|
533 (93%)
|
966 (91%)
|
17 (94%)
|
78 (94%)
|
1594 (92%)
|
|
Referred to toxicologist
|
22 (4%)
|
35 (3%)
|
0
|
3 (3%)
|
60 (3%)
|
|
Co-ingestants
|
|
|
|
|
|
|
Illicit
|
49 (9%)
|
133 (13%)
|
7 (39%)
|
8 (10%)
|
197 (11%)
|
|
Non-illicit
|
73 (13%)
|
312 (29%)
|
9 (50%)
|
60 (72%)
|
454 (26%)
|
|
Route of exposure
|
|
|
|
|
|
|
Ingestion
|
557 (97%)
|
987 (93%)
|
18 (100%)
|
81 (98%)
|
1643 (95%)
|
|
Parenteral
|
15 (3%)
|
56 (5%)
|
0
|
1 (1%)
|
72 (4%)
|
|
Inhaled/nasal
|
3 (1%)
|
16 (2%)
|
0
|
1 (1%)
|
20 (1%)
|
|
Symptom assessment
|
|
|
|
|
|
|
Symptomatic
|
352 (61%)
|
626 (59%)
|
14 (77%)
|
45 (54%)
|
1037 (60%)
|
|
Asymptomatic
|
143 (25%)
|
286 (27%)
|
3 (17%)
|
24 (29%)
|
456 (26%)
|
|
Unknown
|
80 (14%)
|
147 (14%)
|
1 (6%)
|
14 (17%)
|
242 (14%)
|
|
|
All percentages are column percentages, with the exception of the first row (number of reports).
|
Box 2 –
Intentional exposures to methylphenidate, dexamphetamine, modafinil, atomoxetine and methamphetamine reported to the New South Wales Poisons Information Centre, 2004–2014
Box 3 –
Pharmaceutical Benefits Scheme prescriptions for dexamphetamine, methylphenidate, modafinil and atomoxetine, expressed in defined daily doses (DDD)/1000/day, 2004–2014
Box 4 –
Results of joinpoint regression analysis of trends in exposures to methylphenidate, dexamphetamine and methamphetamine reported to the New South Wales Poisons Information Centre, 2004–2014
|
|
Time segment
|
AAPC or APC (95% CI)
|
|
|
Methylphenidate
|
|
|
|
Exposures
|
2004–2014
|
AAPC, 9.8% (7.5 to 12.3%)*
|
|
Dispensing
|
2004–2014
|
AAPC, 10.8% (10.0 to 11.5%)*
|
|
|
2004–2008
|
APC, 15.7% (13.8 to 17.6%)*
|
|
|
2008–2014
|
APC, 7.6% (6.7 to 8.6%)*
|
|
Calls adjusted for DDD/1000/day
|
2004–2014
|
AAPC, −2.0% (−4.4 to 2.9%)
|
|
|
2004–2011
|
APC, 4.5% (−1.1 to 10.4%)
|
|
|
2011–2014
|
APC, −15.7% (−31.4 to 3.6%)
|
|
Dexamphetamine
|
|
|
|
Exposures
|
2004–2014
|
AAPC, −6.6% (−10.7 to −2.3%)*
|
|
Dispensing
|
2004–2014
|
AAPC, −1.4% (−3.2 to 0.3%)
|
|
|
2004–2012
|
APC, −2.7% (−3.8 to −1.7%)*
|
|
|
2012–2014
|
APC, 4.1% (−5.8 to 15.1%)
|
|
Calls adjusted for DDD/1000/day
|
2004–2014
|
AAPC, −4.3% (−9.0 to 0.5%)
|
|
Illicit use of ADHD medication†
|
|
|
|
Exposures
|
2004–2014
|
AAPC, 13.8% (8.1% to 19.7%)*
|
|
Methamphetamine
|
|
|
|
Exposures
|
2004–2014
|
AAPC, 23.4% (2.5% to 48.5%)*
|
|
|
2004–2012
|
APC, 2.3% (−8.9% to 14.8%)
|
|
|
2012–2014
|
APC, 161.5% (−9.5% to 665.9%)
|
|
|
AAPC = average annual per cent change; APC = annual per cent change; DDD/1000/day = defined daily dose per 1000 population per day. * APC or AAPC is significantly different from zero (P < 0.05). † Inferred (alcohol or illicit drugs were co-ingested).
|
Box 5 –
Results of joinpoint regression analysis, stratified by age category and sex: trends in exposures to methylphenidate and dexamphetamine, 2004–2014
|
|
AAPC (95% CI)
|
|
|
Methylphenidate
|
|
|
Adults (20–75 years)
|
8.6% (2.1 to 15.4%)*
|
|
Male
|
8.2% (1.0 to 15.8%)*
|
|
Female
|
9.5% (2.6 to 16.8%)*
|
|
Adolescents (15–19 years)
|
13.0% (8.1 to 18.0%)*
|
|
Male
|
8.2% (1.0 to 15.8%)*
|
|
Female
|
9.5% (2.6 to 16.8%)*
|
|
Children (10–14 years)
|
8.9% (3.5 to 14.6%)*
|
|
Male
|
6.0% (−2.7 to 15.5%)
|
|
Female
|
11.4% (6.5 to 16.5%)*
|
|
Dexamphetamine
|
|
|
Adults (20–75 years)
|
−2.8% (−7.9 to 2.5%)
|
|
Male
|
1.2% (−3.9 to 6.6%)
|
|
Female
|
−7.5% (−14 to −0.4%)*
|
|
Adolescents (15–19 years)
|
−11.9% (−21.4 to −1.3%)*
|
|
Male
|
−17.4% (−24.1 to −10.2%)*
|
|
Female
|
−7.6% (−20.8 to 7.9%)
|
|
Children (10–14 years)†
|
−18.1% (−27.7 to −7.2%)*
|
|
Male
|
−21.5% (−30.6 to −11.3%)*
|
|
|
AAPC = average annual per cent change. * APC or AAPC is significantly different from zero (P < 0.05). † Joinpoint analysis not performed for exposures to dexamphetamine in female children (missing data).
|

 more_vert
more_vert