Obesity (body mass index [BMI] ≥ 30 kg/m2) is a growing health problem and is recognised as one of the largest contributors to the chronic burden of disease. Currently, 28% of Australians are obese, placing our nation second for men and fifth for women among OECD (Organisation for Economic Co-operation and Development) countries ranked by prevalence of obesity.1,2 In Australia, an inverse relationship exists between high obesity prevalence and low socioeconomic status; incidence is almost double for areas indexed as the most disadvantaged compared with areas within the highest strata.3 Those living more remotely also have higher obesity rates — 59% versus 32%.2 Among Indigenous Australians, the prevalence of obesity is almost double (34% versus 18%) and that of type 2 diabetes mellitus (T2DM) is triple that of non-Indigenous adults, resulting in sevenfold greater mortality due to diabetes.2,4
Bariatric surgery is an effective treatment of severe obesity (class III [BMI ≥ 40 kg/m2] or class II [BMI ≥ 35 kg/m2] with comorbid conditions) and is purportedly cost-effective, compared with conservative measures.5–7 The evidence supports sustained postoperative weight loss, ameliorating obesity-related comorbid conditions. The Swedish Obesity Study showed a marked reduction in hypertriglyceridaemia, T2DM and hyperuricaemia with surgery after 2 and 10 years.6 The resultant postsurgical weight loss substantially reduces, resolves and even prevents the metabolic complications associated with increasing central adiposity, with 73%–95% T2DM remission rates by 2 years, depending on the type of surgery.8,9
The heavy economic burden of obesity and its comorbid conditions may be alleviated in the long term by surgical management, despite upfront resource costs.10 Severely obese individuals incur twofold higher mean annual health care costs ($2788 v $1472) and use double the number of medications annually (11.4 v 5.3 per person) compared with the general population.7,11 Weight loss surgery can reduce the number of medications required and lower individual health care costs by 26%, a direct saving of $506 per person.7,12 With evidence of reduced mortality and an acceptably low complication profile (estimated mean 30-day mortality < 0.3%), bariatric procedures are supported by national and international health bodies.13–15
The National Health and Medical Research Council (NHMRC) 2013 guidelines recommend bariatric surgery as the most beneficial and cost-effective management for motivated individuals with severe obesity.16 Motivation is an essential factor in considering whether an individual with obesity and comorbid conditions is a suitable candidate for surgery. Surgery is not the final step in the clinical pathway of severe obesity management; postoperative commitment to lifestyle change and regular follow-up are requisite for successful weight loss and continued improvement in health.16 Most individuals are motivated to have surgery for greater control of medical ailments, yet it is unknown whether this applies to those whose procedure is fully funded.17
Since 1992, Medicare has reimbursed the cost of bariatric surgery in the private sector. As most surgery is carried out in private hospitals with large out-of-pocket expenses for those without private health insurance, a significant inequity in obesity management exists.18 Paradoxically, this surgery is least accessible to those who are likely to be in greatest need. Since October 2009, a pilot program has been underway in the Sydney and South Western Sydney Local Health Districts in which bariatric surgery has been publicly funded for a limited number of patients meeting strict inclusion criteria. There are no Australian studies to date that have identified if publicly funded surgical intervention for severe obesity confers the same health benefits seen in private health care. This study aims to assess the efficacy of bariatric surgery for such patients in the Australian public health system.
Methods
Study design
Sixty-eight moderately to severely obese participants with comorbid conditions were deemed eligible for bariatric surgery, and inclusion in our study. Participants were attendees at an ongoing collaborative pilot program run by one of three obesity clinics based within the Sydney and South Western Sydney Local Health Districts. All participants received conservative management for their obesity and related health conditions delivered by a multidisciplinary team trained in obesity management. Participants were seen 6- to 12-weekly for dietary advice, behavioural modification, advice on physical activity and, where appropriate, very low energy diets (VLEDs) and pharmacotherapy. Participants with substantial obesity-related comorbid conditions who had not achieved adequate weight reduction with conservative intervention, and who were interested in surgical management, were assessed by their multidisciplinary team to determine suitability for bariatric surgery. Longitudinal data were collected on those individuals who underwent bariatric surgery for management of resistant obesity and associated comorbid conditions.
The inclusion criteria for bariatric surgery were: age 18–75 years, minimum class II obesity (BMI ≥ 35 kg/m2) with comorbid conditions, completion of at least 1 year of medical intervention during which the participant had demonstrated commitment to lifestyle change, and surgery before 31 May 2013 (enabling a minimum follow-up period of 3 months). Exclusion criteria were: inability to consent, irreversible endocrine causes of obesity and significant comorbid conditions that were expected to result in poor outcomes from surgery, such as unstable cardiovascular disease and uncontrolled psychiatric illness. Participants underwent 2 weeks of VLED before the surgery to reduce abdominal adiposity and liver volume in particular, as liver size can complicate surgical access.19 They were also instructed about the necessary commitment to postoperative care, specifically the importance of nutritional input. Two of us (C J T and D J M) performed either laparoscopic sleeve gastrectomy (LSG) or laparoscopic adjustable gastric banding (LAGB) at a single public hospital. The type of surgery performed was decided by the participant in conjunction with the surgeon, after being briefed on the techniques, risks and expected benefits of each procedure. All participants were screened for nutritional deficiencies before and after surgery.
Ethics approval was obtained from four Human Research Ethics Committees within the Sydney and South Western Sydney Local Health Districts. All participants gave written consent for use of their records for research purposes.
Clincial and biochemical assessments
Surgeries were performed on an as-needed basis from 1 October 2009 to 31 May 2013. Pre- and postoperative results were analysed at seven time points until 1 September 2013. The baseline (preoperative) measurements were recorded at the start of the VLED, at least 2 weeks before surgery (time zero). Postoperative data were recorded from follow-up appointments at 3, 6, 12, 18, 24 and 36 months. The primary end points analysed were weight, BMI and waist circumference, and secondary end points were four common obesity-related comorbid conditions: T2DM, dyslipidaemia, hypertension (HTN), and obstructive sleep apnoea (OSA). Improvement was defined by normalisation of laboratory markers (T2DM and dyslipidaemia), blood pressure sphygmomanometry (HTN) and polysomnography results (OSA). Comorbid conditions were considered “partially resolved” when participants’ measurements fell within normal limits, the number of medications was reduced or the use of the continuous positive airway pressure (CPAP) device was discontinued. Comorbid conditions were deemed “fully resolved” when normal measurements remained after all relevant medications were discontinued.
Definition of comorbid conditions
T2DM was defined as fasting blood glucose levels ≥ 7 mmol/L, glycated haemoglobin level ≥ 6.5% and/or requirement of at least one oral hypoglycaemic agent. HTN was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg and/or requirement of at least one antihypertensive agent. Dyslipidaemia was defined as total cholesterol level ≥ 5.0 mmol/L, low-density lipoprotein level ≥ 3.5 mmol/L, triglyceride level ≥ 2.0 mmol/L, or high-density lipoprotein level ≤ 1.01 mmol/L for men and ≤ 1.3 mmol/L for women, and/or requirement of at least one lipid-lowering medication.20,21 OSA was defined according to symptoms (snoring, observed apnoea, daytime somnolence), treated HTN and the apnoea–hypopnoea index record from polysomnography.
Statistical analysis
The results at the seven time points were reported as mean and SD. The differences were tested for significance using paired t tests for continuous variables and the McNemar test for paired categorical variables. The t test was used to evaluate between-surgery differences in mean weight at baseline and subsequent time points, to assess the validity of combining the two surgery types in analyses. Mixed-model regression was used to determine if significant change in weight occurred over time at all seven time points, to account for reductions in sample size with time. All statistical analysis was performed using SAS version 9.3 for Windows (SAS Institute).
Results
Participant characteristics
Of the 68 participants offered surgery, two declined and one moved interstate. Sixty-five participants (41 women and 24 men) with a mean age of 51.5 years (SD, 11.8 years; range, 21–73 years) underwent bariatric surgery between 1 October 2009 and 31 May 2013. The level of comorbid conditions in the group at baseline was high, with a mean of eight conditions per patient and class III (severe) obesity. From baseline to 18 months, only three participants were lost to follow-up at each time point; however, numbers are lower with time as operations were done at different times (ie, only 10 patients had surgery 36 months ago whereas 65 had surgery 3 months ago, 58 had surgery 6 months ago, etc [see Box 1]). Data on all participants who completed follow-up at each respective time point according to surgery date are presented in Box 1 and Box 2.
Intraoperative outcomes
All 65 surgeries were performed laparoscopically and none required conversion to open surgery. The most commonly performed procedure was LSG (57 patients; versus eight patients who had LAGB). Despite it appearing that greater weight change occurred in the LSG group, the difference was not significant at 3 months (P = 0.58), 12 months (P = 0.25) or 24 months (P = 0.17). There were no significant intraoperative complications (one participant had a haematoma that resolved with immediate evacuation) and length of hospital stay was within expected times for all patients (LSG, three nights; LAGB, one night).
Short-term postsurgical outcomes (3–6 months)
There was a reduction in all primary end points in the early postoperative period. By 3 months there was a mean weight reduction of 22.6 kg (SD, 9.5 kg) (Box 3) with a 7.4 kg/m2 mean reduction in BMI and 14.5 cm mean reduction in waist circumference (Box 1). All comorbid conditions showed partial resolution from 3 months (Box 2). Of the numbers of participants who had each comorbid condition at baseline and who had data available, there was full resolution by 6 months in 20/45 with T2DM, 14/43 with HTN, 7/47 with dyslipidaemia and 17/41 with OSA (Box 2). The requirement for all relevant medications also reduced accordingly (Box 4).
Medium-term postsurgical outcomes (12–18 months)
By 1 year, there was a significant reduction in all anthropometric measures. The mean weight loss was 34.2 kg (SD, 20.1 kg), and the mean BMI was reduced by 12 kg/m2 to 36.2 kg/m2 (SD, 7.7 kg/m2). By 18 months, of those who reported the respective comorbid conditions at baseline, almost half of the group had full remission of T2DM and three-quarters had resolution of OSA. Modest resolution of dyslipidaemia was seen with both a significant rise in high-density lipoprotein and reduction in triglycerides occurring transiently at 6–12 months in 7 of 47 patients who had dyslipidaemia at baseline (P < 0.001).
Long-term postsurgical outcomes (24–36 months)
The trend for continued weight loss and partial or full resolution of comorbid conditions appears maximal at 12–24 months. While the smaller group sizes at the 24- and 36-month follow-ups provided less power for statistical analysis, primary and secondary end points remained stable (P < 0.001). The maximal amount of mean weight loss occurred at 24 months (39.9 kg; SD, 31.4 kg). Three-quarters of the cohort had reduced the number of medications taken for HTN and T2DM by 18–24 months, and by 36 months, all had reduced the number of antihypertensive medications (Box 4).
Postoperative complications
Transient nutritional deficiencies were found in three of 65 participants, improving within 3 months with replacement treatment. Of the eight patients who had LAGB, one underwent conversion, due to gastric band erosion, to a Roux-en-Y bypass at 18 months. Of the participants who had LSG, one had a small bowel obstruction secondary to adhesions and required laparoscopic division at 2 years. Three participants required endoscopic stomach dilatation and a further three continued to experience nausea, dyspepsia and/or vomiting at 3 months, but this resolved by 6 months for two and at 12 months for one participant.
Weight change over time
A total of 288 observations for weight from 65 participants were available for linear mixed-model analysis. Significant change in mean weight was observed over time (P < 0.001). Mean weight adjusted for baseline weight decreased from 136.5 kg at baseline to 101.1 kg at 12 months. Stratifying the analysis by sex showed that men had a higher mean baseline weight, 147.5 kg (SD, 36.9 kg) compared with women, 130.1 kg (SD, 23.8 kg), but no difference was observed after 1 year (Box 3).
Discussion
In our study, the significant weight lost by obese participants who underwent bariatric surgery occurred early and was sustained over the first 3 years. The mean maximal weight loss was almost one-third of participants’ preoperative weight and was achieved by 24 months. Despite decreasing sample sizes, our results showed sustained weight loss at 36 months.
There was full or partial resolution of all comorbid conditions tested, except dyslipidaemia, in most participants by 2 years. Different rates of resolution of comorbid conditions occurred, with remission of T2DM being the earliest. By 24 months, there was an associated resolution of T2DM, HTN and OSA, or the need for pharmacotherapy or devices was reduced in most patients, yet dyslipidaemia showed inconclusive results. These findings parallel other bariatric studies.22,23 The Swedish Obesity Study found isolated improvements in hypertriglyceridaemia postoperatively (including after LAGB), although the most consistent improvements in lipid profiles have been seen after Roux-en-Y bypass surgery.6,24
Most LAGB procedures were performed in the first year of the study, until LSG became a preferred option due to its greater weight loss profile with minimal additional complications and the lower need for surgical follow-up. However, throughout the study, participants had autonomy of procedure choice (on surgeon recommendation). While this may suggest an avenue for bias (the participants chose their “intervention”), bariatric surgery is an elective procedure, and it was deemed necessary to replicate the protocol in the private sector. The limitations of our study include its small size, that it is a case series, that it is non-blinded, possible bias due to participants choosing their surgery type, and the use of a clinical database with the possibility of missing or inaccurate values imputed by health professionals. The low rates of adverse events observed in our study are consistent with those reported in the literature.
The direct operative costs of performing these surgeries in the public sector in our study were estimated to be $7000–$9000 (LSG incurred greater up-front theatre costs than LAGB). Perioperative costs, including 2 years of postsurgical visits, may approach $2000 per person, taking the total cost to $9000–$11 000 per person. A 2005 paper reported the annual cost of managing an individual with T2DM as $9095–$15 850.25 Thus, if an obese person with T2DM has bariatric surgery, the operation would pay for itself after about 1 year.
The participants’ baseline characteristics are representative of a typical demographic seen in the public sector, supporting extrapolation of our results to a wider population. The high attendance rate at 2 years shows that the cohort demonstrated adequate motivation to justify surgical intervention. The health potential from bariatric surgery ranges from improved quality of life and amelioration of comorbid conditions to full resolution of complications and reduced mortality for all individuals, paying or not. Strategies to prioritise access are therefore recommended to reduce the apparent inequality that exists. Limited access to surgery discriminates against those who cannot afford the out-of-pocket costs, yet it is likely that this subgroup would benefit most. In conclusion, we hope that our study provides an evidence base for the surgical treatment of obesity in the public health system and, in turn, that consideration will be given to increasing the supply of publicly funded bariatric surgery in Australia.
1 Patient anthropometric data before and after bariatric surgery
|
Measurement |
Baseline (2 weeks before surgery) |
Postoperative month 3 |
Postoperative month 6 |
Postoperative month 12 |
Postoperative month 18 |
Postoperative month 24 |
Postoperative month 36 |
||||||||
|
|
|||||||||||||||
|
N (n) |
65 (65) |
65 (65) |
55 (58) |
49 (52) |
30 (33) |
17 (23) |
7 (10) |
||||||||
|
BMI (SD), kg/m2 |
48.2 (9.5) |
40.8 (8.3)* |
38.9 (7.9)* |
36.2 (7.7)* |
38.2 (12.1)* |
35.7 (7.7)* |
38.7 (9.4)† |
||||||||
|
Weight (SD), kg |
136.5 (30.3) |
113.9 (25.2)* |
108.3 (24.8)* |
101.1 (22.4)* |
99.3 (20.8)* |
97.6 (21.9)* |
108.6 (25.6)† |
||||||||
|
Waist circumference (SD), cm |
132.2 (16.5) |
117.7 (15.2)* |
114.9 (14.7)* |
109.4 (13.7)* |
110.2 (12.3)* |
108.4 (15.8)* |
114.9 (14.6)* |
||||||||
|
% weight loss from baseline |
0 |
17% |
21% |
26% |
27% |
29% |
21% |
||||||||
|
|
|||||||||||||||
|
BMI = body mass index. N = total number of participants who attended follow-up. n = total number of participants including those lost to follow-up. * Comparison between baseline and follow-up; P < 0.001. † Comparison between baseline and follow-up; P < 0.05. |
|||||||||||||||
2 Proportions of patients* who continued to have conditions found at baseline
|
Comorbid condition |
Baseline (2 weeks before surgery) |
Postoperative month 3 |
Postoperative month 6 |
Postoperative month 12 |
Postoperative month 18 |
Postoperative month 24 |
Postoperative month 36 |
||||||||
|
|
|||||||||||||||
|
T2DM |
53 |
36/52† |
25/45† |
21/42† |
12/23† |
8/17‡ |
3/6 |
||||||||
|
HTN |
51 |
45/51‡ |
29/43† |
18/40† |
10/27† |
4/12‡ |
2/6‡ |
||||||||
|
Dyslipidaemia |
58 |
45/58‡ |
40/47 |
33/42 |
13/24‡ |
8/15‡ |
4/6 |
||||||||
|
OSA |
41 |
37/41 |
24/41‡ |
15/41† |
6/24† |
4/17‡ |
2/5 |
||||||||
|
OSA requiring CPAP |
27 |
18/27 |
14/23‡ |
8/22† |
4/13‡ |
2/8‡ |
1/3 |
||||||||
|
|
|||||||||||||||
|
CPAP = continuous positive airway pressure. HTN = hypertension. OSA = obstructive sleep apnoea. T2DM = type 2 diabetes mellitus. * Numerators are number of participants who had that condition at that time point. Denominators are total number of participants who had that condition at baseline and had data at that time point. † Comparison between baseline and follow-up; P < 0.001. ‡ Comparison between baseline and follow-up; P < 0.05. |
|||||||||||||||
3 Patients’ weight change over time after bariatric surgery*
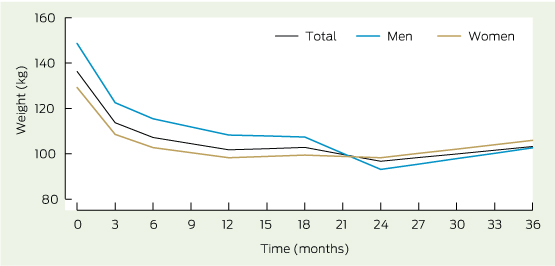
* Weight change at all seven time points is significant (P < 0.001). Despite differences in weight lost for men and women, there were no significant between-sex differences.
4 Number of patients with a reduction in medications used at each time point after bariatric surgery
|
Antihypertensive medications |
Glucose-lowering medications |
Lipid-lowering medications |
|||||||||||||
|
Postoperative month |
Patients at |
Patients with reduced use of medication |
Patients at each time point |
Patients with reduced use |
Patients at each time point |
Patients with reduced use |
|||||||||
|
|
|||||||||||||||
|
3 |
65 |
31 |
60 |
28 |
64 |
6 |
|||||||||
|
6 |
54 |
34 |
54 |
29 |
53 |
10 |
|||||||||
|
12 |
48 |
37 |
47 |
25 |
44 |
12 |
|||||||||
|
18 |
29 |
22 |
23 |
17 |
24 |
12 |
|||||||||
|
24 |
16 |
12 |
16 |
15 |
13 |
3 |
|||||||||
|
36 |
6 |
6 |
6 |
4 |
4 |
1 |
|||||||||

 more_vert
more_vert