He is vain, angry, self-important, and has sagging, elderly buttocks: Henry Marsh tells us this about himself in his new book Admissions: A Life In Brain Surgery—honest to the point of brutal. This is the life and times of a famous neurosurgeon, entering retirement, who at once seems to be delighted by his renown but also despises it. Marsh names people that he has bitterly fallen out with; shamefully documents how he pulled the nose of a nurse over a dispute about a nasogastric tube; describes his own treatment by a psychiatrist; spells out his guilty mistakes in operating; and describes how, if terminally ill, he would regard palliative care specialists as professionals who “derive their own sense of meaning and purpose from my suffering”.
Preference: Surgery
1175
“Are you in the market for a hardtop?”
BY DR CLIVE FRASER
Cyclone Debbie versus three Volvos
At 2PM on Tuesday 28th March 2017 Tropical Cyclone Debbie crossed the Queensland coastline at Airlie Beach.
With wind speeds of 190 km/h and peak gusts of up to 270 km/h there was always going to be a lot of damage from the Category 4 cyclone, and the popular resort islands of the Whitsundays were hardest hit.
At the moment that Cyclone Debbie crossed the Queensland coast my surgery was over 1,000 km to the SSE and I was still enjoying sunny skies.
I’d just left my beloved 1997 Volvo V70 wagon at a local repairer for some maintenance.
Having learnt to ignore whatever politicians say and being unable to read the sign language, I didn’t heed Wednesday’s warnings from our Premier to stay at home all day on Thursday.
But I did try to postpone my bookings, all to no avail.
By 5PM I’d finished my clinic to start on paperwork and my dictation.
At that moment, the power went out.
I soldiered on with a torch, but then decided it was time to go home, only to find that my loan car was trapped in the car park as the electric gate wouldn’t open.
Two hours later and I was finally on my way.
The worst of the bad weather was over, but there were trees down everywhere.
By Friday morning there were clear skies again, so I decided to recover my V70.
I wasn’t really ready for the damage I encountered at the mechanical workshop when I discovered that a 30 metre tall gum tree had fallen in the storm on top of at least three Volvos.
Like an anxious parent I scanned the yard for my car.
I couldn’t see it among the foliage. Surely it wasn’t under the mass of branches in front of me?
After 20 years of ownership would I finally be saying goodbye to my V70.
Well the anxiety was unwarranted because my car was intact some distance from the fallen trunk.
But how did the other Volvos fare under such a mass of wood and leaves?
Surprisingly well was my observation.
They all had broken windscreens, front and back.
The roofs were dented, but none were crushed.
And yes, true to Volvo’s claims, all of the doors still opened.
The scene reminded me of that wonderful 1971 Volvo advertisement which showed seven Volvo 140’s stacked on top of one another.
The theory was that Volvos had six steel pillars supporting the roof and that each one could support the entire weight of the vehicle.
On paper, six cars could be stacked on top of a Volvo.
It was a marketing masterpiece when Volvo was being criticized for boxy styling and staid dynamics.
Fast forward to today and there is still no ANCAP fallen gum tree crush test.
If there was I could confirm that all of the Volvos would have passed.
So I thought that I would introduce more real world testing into this column.
I’ll be looking in particular at how the technology in modern cars enables them to avoid collisions with feral pigs, and what happens when one hits a kangaroo.
Please send your stories of other non-ANCAP collisions to doctorclivefraser@hotmail.com.
Safe motoring,
Doctor Clive Fraser
doctorclivefraser@hotmail.com
Bertel Sunstrup 24.1.1931 / 22.4.2017
OBITUARY
Bertel Sunstrup 24.1.1931 / 22.4.2017
On the 24th of January 1931, Bert was born in Wondai Queensland. His early days were spent in Gympie.
He went to ‘Shore’ Grammar School in Sydney and graduated MBBS at Sydney University. He did his residency in Launceston and Hobart Hospital before joining Dr Gunson at the Northern Suburbs Medical Clinic in 1958. During this time he (like many GPs) also gave the anaesthetics for the surgeons in both the private and public hospitals. Bert then accepted the Registrar job for the Launceston branch of the Peter Mac Callum Radiotherapy Unit working with Dr Harry Holden.
After a few years he went to England and obtained his ‘Radiotherapy/Oncology’ qualification. Bert returned to the Launceston Hospital to work with Dr Holden and then took over the Radiotherapy/Oncology unit when Dr Holden retired. In 1986 the unit name was changed to the Holman Clinic after its founder in 1928-32.
In his profession Bert witnessed a lot of pain, despair and suffering on a daily basis. He was a dedicated and inspirational clinician who always listened with compassion to his patients being mindful of their difficulties especially in coping with everyday challenges with cancer.
In the 1990s Bert “fought Tooth and Nail” to stop the bureaucrats from transferring the Radiotherapy/Oncology Unit to Hobart. He was steadfastly determined and presented irrefutable arguments that we must continue to treat the patients with all forms of cancers in the North of Tasmania. Were it not for him there would be no clinic in the North. Thankfully the Government agreed to keep the Holman Clinic at the Launceston General Hospital
In 1983 Bert purchased a farm in Pipers Brook and started a vineyard with the help of his wife Anne, her sister Jill and son Christopher. His wine ‘Dalrymple’ soon became well received and they won many medals at the wine shows.
Bert’s other significant interest was skiing. When he returned to Tasmania he married Anne, a registered nurse, had three children. They built their own shack in the Ben Lomond Ski Village. He was a wonderfully entertaining, witty and generous man who had some great parties in their shack. Bert and Anne soon joined the Ben Lomond Ski Patrol and he was promoted from Patrol Doctor to President and eventually to Life Member. Once again it was his infectious energy and enthusiasm that encouraged many to join the patrol and keep the skiers safe and, if injured, to provide them with the correct treatment before they left the mountain.
I loved talking with Bert we shared the same values and had similar aspirations and concerns. He was better informed than I in history and would constantly come up with some interesting trivia.
All past, present and future patients in the North of Tasmania and in particular the Launceston General Hospital are indebted to this friendly, unassuming and dedicated man.
I am certain that his children; Katrina, Ingrid and Christopher as well as his medical colleagues will keep his spirit and legacy alive.
Professor Berni Einoder A.M.
Director of Surgery at LGH 1984 to 2014
Neurobionics and the brain–computer interface: current applications and future horizons
Neurobionics is the science of directly integrating electronics with the nervous system to repair or substitute impaired functions. The brain–computer interface (BCI) is the linkage of the brain to computers through scalp, subdural or intracortical electrodes (Box 1). Development of neurobionic technologies requires interdisciplinary collaboration between specialists in medicine, science, engineering and information technology, and large multidisciplinary teams are needed to translate the findings of high performance BCIs from animals to humans.1
Neurobionics evolved out of Brindley and Lewin’s work in the 1960s, in which electrodes were placed over the cerebral cortex of a blind woman.2–4 Wireless stimulation of the electrodes induced phosphenes — spots of light appearing in the visual fields. This was followed in the 1970s by the work of Dobelle and colleagues, who provided electrical input to electrodes placed on the visual cortex of blind individuals via a camera mounted on spectacle frames.2–4 The cochlear implant, also developed in the 1960s and 1970s, is now a commercially successful 22-channel prosthesis for restoring hearing in deaf people with intact auditory nerves.5 To aid those who have lost their auditory nerves, successful development of the direct brainstem cochlear nucleus multi-electrode prosthesis followed.6
The field of neurobionics has advanced rapidly because of the need to provide bionic engineering solutions to the many disabled US veterans from the Iraq and Afghanistan wars who have lost limbs and, in some cases, vision. The United States Defense Advanced Research Projects Agency (DARPA) has focused on funding this research in the past decade.7
Through media reports about courageous individuals who have undergone this pioneering surgery, disabled people and their families are becoming more aware of the promise of neurobionics. In this review, we aim to inform medical professionals of the rapid progress in this field, along with ethical challenges that have arisen. We performed a search on PubMed using the terms “brain computer interface”, “brain machine interface”, “cochlear implants”, “vision prostheses” and “deep brain stimulators”. In addition, we conducted a further search based on reference lists in these initial articles. We tried to limit articles to those published in the past 10 years, as well as those that describe the first instances of brain–machine interfaces.
Electrode design and placement
Neurobionics has been increasing in scope and complexity because of innovative electrode design, miniaturisation of electronic circuitry and manufacture, improvements in wireless technology and increasing computing power. Using computers and advanced signal processing, neuroscientists are learning to decipher the complex patterns of electrical activity in the human brain via these implanted electrodes. Multiple electrodes can be placed on or within different regions of the cerebral cortex, or deep within the subcortical nuclei. These electrodes transmit computer-generated electrical signals to the brain or, conversely, receive, record and interpret electrical signals from this region of the brain.
Microelectrodes that penetrate the cortical tissue offer the highest fidelity signals in terms of spatial and temporal resolution, but they are also the most invasive (Box 2, A).8 These electrodes can be positioned within tens of micrometres of neurons, allowing the recording of both action potential spikes (the output) of individual neurons and the summed synaptic input of neurons in the form of the local field potential.9 Spiking activity has the highest temporal and spatial resolution of all the neural signals, with action potentials occurring in the order of milliseconds. In contrast, the local field potential integrates information over about 100 μm, with a temporal resolution of tens to hundreds of milliseconds.
Electrocorticography (ECoG), using electrodes placed in the subdural space (on the cortical surface), and electroencephalography (EEG), using scalp electrodes, are also being used to detect cortical waveforms for signal processing by advanced computer algorithms (Box 2, C, D). Although these methods are less invasive than using penetrating microelectrodes, they cannot record individual neuron action potentials, instead measuring an averaged voltage waveform over populations of thousands of neurons. In general, the further away the electrodes are from the brain, the safer the implantation procedure is, but with a resulting decrease in the signal-to-noise ratio and the amount of control signals that can be decoded (ie, there is a lot of background noise). Therefore, ECoG recordings, which are closer to the brain, typically have a higher signal spatial and temporal resolution than that achievable by EEG.8 As EEG electrodes are placed on the opposite side of the skull from the brain, the recordings have a low fidelity and a low signal-to-noise ratio. For stimulation, subdural electrodes require higher voltages to activate neurons than intracortical electrodes and are less precise for stimulation and recording. Transcranial magnetic stimulation can be used to stimulate populations of neurons, but this is a crude technique compared with the invasive microelectrode techniques.10
Currently, implanted devices have an electrical plug connection through the skull and scalp, with attached cables. This is clearly not a viable solution for long term implantation. The challenge for engineers has been to develop the next generation of implantable wireless microelectronic devices with a large number of electrodes that have a long duration of functionality. Wireless interfaces are beginning to emerge.3,11–13
Applications for brain–computer interfaces
Motor interfaces
The aim of the motor BCI has been to help paralysed patients and amputees gain motor control using, respectively, a robot and a prosthetic upper limb. Non-human primates with electrodes implanted in the motor cortex were able, with training, to control robotic arms through a closed loop brain–machine interface.14 Hochberg and colleagues were the first to place a 96-electrode array in the primary motor cortex of a tetraplegic patient and connect this to a computer cursor. The patient could then open emails, operate various devices (such as a television) and perform rudimentary movements with a robotic arm.15 For tetraplegic patients with a BCI, improved control of the position of a cursor on a computer screen was obtained by controlling its velocity and through advanced signal processing.16 These signal processing techniques find relationships between changes in the neural signals and the intended movements of the patient.17,18
Reach, grasp and more complex movements have been achieved with a neurally controlled robotic arm in tetraplegic patients.19,20 These tasks are significantly more difficult than simple movements as they require decoding of up to 15 independent signals to allow a person to perform everyday tasks, and up to 27 signals for a full range of movements.21,22 To date, the best BCI devices provide fewer than ten independent signals. The patient requires a period of training with the BCI to achieve optimal control over the robotic arm. More complex motor imagery, including imagined goals and trajectories and types of movement, has been recorded in the human posterior parietal cortex. Decoding this imagery could provide higher levels of control of neural prostheses.23 More recently, a quadriplegic patient was able to move his fingers to grasp, manipulate and release objects in real time, using a BCI connected to cutaneous electrodes on his forearms that activated the underlying muscles.24
The challenge with all these motor cortex electrode interfaces is to convert them to wireless devices. This has recently been achieved in a monkey with a brain–spinal cord interface, enabling restoration of movement in its paralysed leg,25 and in a paralysed patient with amyotrophic lateral sclerosis, enabling control of a computer typing program.11
These examples of BCIs have primarily used penetrating microelectrodes, which, despite offering the highest fidelity signal, suffer from signal loss over months to years due to peri-electrode gliosis.26 This scarring reduces electrical conduction and the resulting signal change can require daily or even hourly recalibration of the algorithms used to extract information.18 This makes BCIs difficult to use while unsupervised and hinders wider clinical application, including use outside a laboratory setting.
A recently developed, less invasive means of electrode interface with the motor cortex is the stent-electrode recording array (“stentrode”) (Box 2, B).27 This is a stent embedded with recording electrodes that is placed into the sagittal venous sinus (situated near the motor cortex) using interventional neuroradiology techniques. This avoids the need for a craniotomy to implant the electrodes, but there are many technical challenges to overcome before human trials of the stentrode can commence.
Lower-limb robotic exoskeleton devices that enable paraplegic patients to stand and walk have generated much excitement and anticipation. BCIs using scalp EEG electrodes are unlikely to provide control of movement beyond activating simple robotic walking algorithms in the exoskeleton, such as “walk forward” or “walk to the right”. Higher degrees of complex movement control of the exoskeleton with a BCI would require intracranial electrode placement.28 Robotic exoskeleton devices are currently cumbersome and expensive.
Sensory interfaces
Fine control of grasping and manipulation of the hand depends on tactile feedback. No commercial solution for providing artificial tactile feedback is available. Although early primate studies have produced artificial perceptions through electrical stimulation of the somatosensory cortex, stimulation can detrimentally interfere with the neural recordings.29 Optogenetics — the ability to make neurons light-sensitive — has been proposed to overcome this.30 Sensorised thimbles have been placed on the fingers of the upper limb myoelectric prosthesis to provide vibratory sensory feedback to a cuff on the arm, to inform the individual when contact with an object is made and then broken. Five amputees have trialled this, with resulting enhancement of their fine control and manipulation of objects, particularly for fragile objects.31 Sensory feedback relayed to the peripheral nerves and ultimately to the sensory cortex may provide more precise prosthetic control.32
Eight people with chronic paraplegia who used immersive virtual reality training over 12 months saw remarkable improvements in sensory and motor function. The training involved an EEG-based BCI that activated an exoskeleton for ambulation and visual–tactile feedback to the skin on the forearms. This is the first demonstration in animals or humans of long term BCI training improving neurological function, which is hypothesised to result from both spinal cord and cortical plasticity.33
The success of the cochlear prosthesis in restoring hearing to totally deaf individuals has also demonstrated how “plastic” the brain is in learning to interpret electrical signals from the sound-processing computer. The recipient learns to discern, identify and synthesise the various sounds.
The development of bionic vision devices has mainly focused on the retina, but electrical connectivity of these electrode arrays depends on the recipient having intact neural elements. Two retinal implants are commercially available.3 Retinitis pigmentosa has been the main indication. Early trials of retinal implants are commencing for patients with age-related macular degeneration. However, there are many blind people who will not be able to have retinal implants because they have lost the retinal neurons or optic pathways. Placing electrodes directly in the visual cortex bypasses all the afferent visual pathways.
It has been demonstrated that electrical stimulation of the human visual cortex produces discrete reproducible phosphenes. Several groups have been developing cortical microelectrode implants to be placed into the primary visual cortex. Since 2009, the Monash Vision Group has been developing a wireless cortical bionic vision device for people with acquired bilateral blindness (Box 3). Photographic images from a digital camera are processed by a pocket computer, which transforms the images into the relevant contours and shapes and into patterns of electrical stimulation that are transmitted wirelessly to the electrodes implanted in the visual cortex (Box 3, B). The aim is for the recipient to be able to navigate, identify objects and possibly read large print. Facial recognition is not offered because the number of electrodes will not deliver sufficient resolution.2 A first-in-human trial is planned for late 2017.2,34
The lateral geniculate nucleus of the thalamus is an alternative site for implantation of bionic vision devices. Further technical development of the design, manufacture and placement of multiple brain microelectrodes in this small deep brain structure is needed before this could be applied in humans.35
Memory restoration and enhancement
The same concepts and technologies used to record and stimulate the brain in motor and sensory prostheses can also be applied to deeper brain structures. For example, the fornix is an important brain structure for memory function. A human safety study of bilateral deep brain stimulation of the fornix has been conducted in 42 patients with mild, probable Alzheimer disease (ADvance trial), and this study will now proceed to a randomised controlled trial.36 This technique involves deep brain stimulation without direct feedback from neural recording.
A more definitive approach to memory augmentation would be to place a multi-electrode prosthesis directly into the hippocampus. Electrical mimicry of encoded patterns of memory about a task transmitted from trained donor rats to untrained recipient rats resulted in enhanced task performance in the recipients.37,38 This technology has been applied to the prefrontal cortex of non-human primates.39 Although human application is futuristic, this research is advancing rapidly. A start-up company was formed in 2016 to develop this prosthetic memory implant into a clinic-ready device for people with Alzheimer disease.40 The challenge in applying these therapies to Alzheimer disease and other forms of dementia will be to intervene before excessive neuronal loss has occurred.
Seizure detection and mitigation
Many patients with severe epilepsy do not achieve adequate control of seizures with medication. Deep brain electrical stimulation, using electrodes placed in the basal ganglia, is a treatment option for patients with medically refractory generalised epilepsy.41 Methods to detect the early onset of epileptic seizures using cortical recording and stimulation (to probe for excitability) are evolving rapidly.42 A hybrid neuroprosthesis, which combines electrical detection of seizures with an implanted anti-epileptic drug delivery system, is also being developed.43,44
Parkinson disease and other movement disorders
Deep brain stimulation in the basal ganglia is an effective treatment for Parkinson disease and other movement disorders.45 This type of BCI includes a four-electrode system implanted in the basal ganglia, on one or both sides, which is connected to a pulse generator implanted in the chest wall. This device can be reprogrammed wirelessly. Novel electrodes with many more electrode contacts and a recording capacity are being developed. This feedback controlled or closed loop stimulation will require a fully implanted BCI, so that the deep brain stimulation is adaptive and will better modulate the level of control of the movement disorder from minute to minute. More selective directional and steerable deep brain stimulation, with the electrical current being delivered in one direction from the active electrodes, rather than circumferentially, is being developed. The aim is to provide more precise stimulation of the target neurons, with less unwanted stimulation of surrounding areas and therefore fewer side effects.46
Technical challenges and future directions
Biocompatibility of materials, electrode design to minimise peri-electrode gliosis and electrode corrosion, and loss of insulation integrity are key engineering challenges in developing BCIs.47 Electrode carriers must be hermetically sealed to prevent ingress of body fluids. Smaller, more compact electronic components and improved wireless interfaces will be required. Electronic interfaces with larger numbers of neurons will necessitate new electrode design, but also more powerful computers and advanced signal processing to allow significant use time without recalibration of algorithms.
Advances in nanoscience and wireless and battery technology will likely have an increasing impact on BCIs. Novel electrode designs using materials such as carbon nanotubes and other nanomaterials, electrodes with anti-inflammatory coatings or mechanically flexible electrodes to minimise micromotion may have greater longevity than standard, rigid, platinum–iridium brain electrodes.48 Electrodes that record from neural networks in three dimensions have been achieved experimentally using injectable mesh electronics with tissue-like mechanical properties.49 Optogenetic techniques activate selected neuronal populations by directing light onto neurons that have been genetically engineered with light-sensitive proteins. There are clearly many hurdles to overcome before this technology is available in humans, but microscale wireless optoelectronic devices are working in mice.50
Populating the brain with nanobots that create a wireless interface may eventually enable direct electronic interface with “the cloud”. Although this is currently science fiction, the early stages of development of this type of technology have been explored in mice, using intravenously administered 10 μg magnetoelectric particles that enter the brain and modify brain activity by coupling intrinsic neural activity with external magnetic fields.51
Also in development is the electrical connection of more than one brain region to a central control hub — using multiple electrodes with both stimulation and recording capabilities — for integration of data and neuromodulation. This may result in more nuanced treatments for psychiatric illness (such as depression, post-traumatic stress disorder and obsessive compulsive disorder), movement disorders, epilepsy and possibly dementia.
Ethical and practical considerations
Implantable BCI devices are in an early phase of development, with most first-in-human studies describing only a single patient. However, the performance of these devices is rapidly improving and, as they become wireless, the next step will be to implant BCIs in larger numbers of patients in multicentre trials.
The prime purpose of neurobionic devices is to help people with disabilities. However, there will be pressure in the future for bionic enhancement of normal cognitive, memory, sensory or motor function using BCIs. Memory augmentation, cognitive enhancement, infrared vision and exoskeletal enhancement of physical performance will all likely be achievable.
The introduction of this technology generates many ethical challenges, including:
-
appreciation of the risk–benefit ratio;
-
provision of adequate and balanced information for the recipient to give informed consent;
-
affordability in relation to the fair and equitable use of the scarce health dollar;
-
inequality of patient access to implants, particularly affecting those in poorer countries;
-
undue influence on physicians and scientists by commercial interests; and
-
the ability to achieve unfair physical or cognitive advantage with the technology, such as enhancing disabled athletes’ performance using exoskeleton devices, military application with the creation of an enhanced “super” soldier, or using a BCI as the ultimate lie detector.52
The introduction of these devices into clinical practice should therefore not proceed unchecked. As the technology transitions from clinical trial to the marketplace, training courses and mentoring will be needed for the surgeons who are implanting these devices. Any new human application of the BCI should be initially tested for safety and efficacy in experimental animal models. After receiving ethics committee approval for human application, the technology should be thoroughly evaluated in well conducted clinical trials with clear protocols and strict inclusion criteria.53
One question requiring consideration is whether sham surgery should be used to try to eliminate a placebo effect from the implantation of a new BCI device. Inclusion of a sham surgery control group in randomised controlled trials of surgical procedures has rarely been undertaken,54 and previous trials involving sham surgery have generated much controversy.55–57 Sham surgery trials undertaken for Parkinson disease have involved placing a stereotactic frame on the patient and drilling of burr holes but not implanting embryonic cells or gene therapy.58–60 We do not believe sham surgery would be applicable for BCI surgery, for several reasons. First, each trial usually involves only one or a few participants; there are not sufficient numbers for a randomised controlled trial. Second, the BCI patients can serve as their own controls because the devices can be inactivated. Finally, although sham controls may be justified if there is likely to be a significant placebo effect from the operation, this is not the case in BCI recipients, who have major neurological deficits such as blindness or paralysis.
Clinical application of a commercial BCI will require regulatory approval for an active implantable medical device, rather than approval as a therapy. It is also important for researchers to ask the potential recipients of this new technology how they feel about it and how it is likely to affect their lives if they volunteer to receive it.61 This can modify the plans of the researchers and the design of the technology. The need for craniotomy, with its attendant risks, may deter some potential users from accepting this technology.
As the current intracortical electrode interfaces may not function for more than a few years because of electrode or device failure, managing unrealistic patient and family expectations is essential. Trial participants will also require ongoing care and monitoring, which should be built into any trial budget. International BCI standards will need to be developed so that there is uniformity in the way this technology is introduced and evaluated.
Conclusions
BCI research and its application in humans is a rapidly advancing field of interdisciplinary research in medicine, neuroscience and engineering. The goal of these devices is to improve the level of function and quality of life for people with paralysis, spinal cord injury, amputation, acquired blindness, deafness, memory deficits and other neurological disorders. The capability to enhance normal motor, sensory or cognitive function is also emerging and will require careful regulation and control. Further technical development of BCIs, clinical trials and regulatory approval will be required before there is widespread introduction of these devices into clinical practice.
Box 1 –
Schematic overview of the major components of brain–computer interfaces
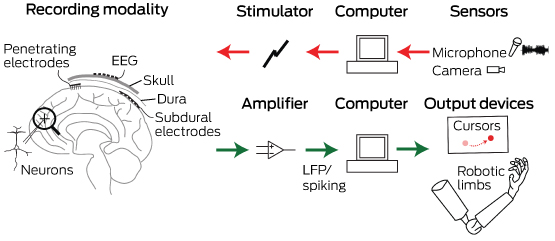
Common to all devices are electrodes that can interface at different scales with the neurons in the brain. For output-type interfaces (green arrows), the brain signals are amplified and control signals from them are decoded via a computer. These decoded signals are then used to control devices that can interact with the world, such as computer cursors or robotic limbs. For input-type interfaces (red arrows), such as vision or auditory prostheses, a sensor captures the relevant input, which a computer translates into stimulation parameters that are sent to the brain via an electrode interface. EEG = electroencephalography. LFP = local field potential.
Box 2 –
Electrodes of different scales that can be used to record neural activity for brain–computer interfaces
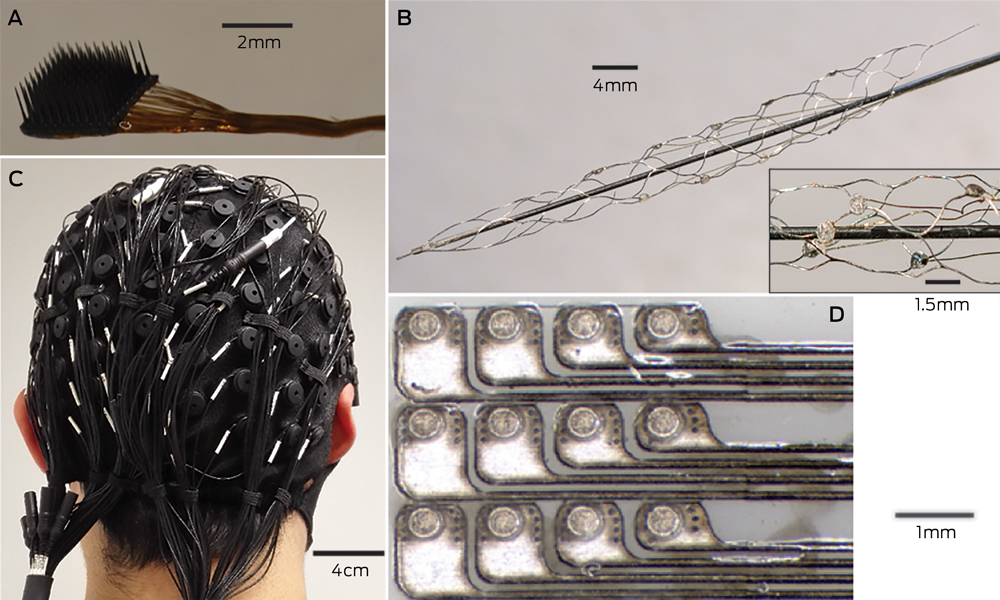
A: The most invasive method of recording neural activity, which produces the best signal quality, requires penetrating microelectrodes, such as this Utah array (Blackrock Microsystems), with 100 electrodes with a spacing of 400 μm. Wires connected to each electrode (bundled to the right of the image) need to be percutaneously connected to the outside world. B: Electrodes placed on an intravascular stent with (inset) a close-up image of a few electrodes (750 μm diameter). C: A 128-channel, non-invasive electroencephalography cap. After the cap is applied to the scalp, conductive gel is injected into each electrode to ensure electrical contact. D: An example of a planar array that can be placed in the subdural space to record electrocorticography signals. The platinum electrodes (350 μm diameter circles) are embedded in silicone.
Box 3 –
An example of a fully implantable brain–computer interface
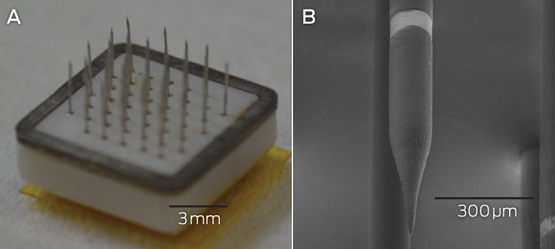
A: The Monash Vision Group cortical vision prosthesis, which consists of an array of penetrating microelectrodes (metallic spikes) connected through a ceramic casing to electronics that are capable of delivering electrical stimulation and receiving wireless power and control signals. B: A close-up of a single electrode with a 150 μm diameter. The bright band is the conductive ring electrode, where electrical charge is delivered. Electrodes are spaced 1 mm apart.
Indexation freeze hits veterans’ health care
A recent survey of some AMA members has highlighted the impact of the Government’s ongoing indexation freeze on access to Department of Veterans’ Affairs (DVA) funded specialist services for veterans.
The DVA Repatriation Medical Fee Schedule (RMFS) has been frozen since 2012.
The AMA conducted the survey following anecdotal feedback from GP and other specialist members that veterans were facing increasing barriers to accessing specialist medical care.
Running between March 3 and 10, the survey was sent to AMA specialist members (excluding general practice) across the country.
It attracted interest from most specialties, although surgery, medicine, anaesthesia, psychiatry and ophthalmology dominated the responses.
More than 98 per cent of the 557 participants said they treat or have treated veterans under DVA funded health care arrangements.
For the small number of members who said they did not, inadequate fees under the RMFS was nominated as the primary reason for refusing to accept DVA cards.
When asked, 79 per cent of respondents said they considered veteran patients generally had a higher level of co-morbidity or, for other reasons, required more time, attention and effort than other private patients.
According to the survey results, the indexation freeze is clearly having an impact on access to care for veterans and this will only get worse over time.
Table 1 highlights that only 71.3 per cent of specialists are currently continuing to treat all veterans under the DVA RMFS, with the remainder adopting a range of approaches including closing their books to new DVA funded patients or treating some as fully private or public patients.
If the indexation freeze continues, the survey confirmed that the access to care for veterans with a DVA card will become even more difficult.
Table 2 shows that less than 45 per cent of specialists will continue to treat all veterans under the DVA RMFS while the remainder will reconsider their participation, either dropping out altogether or limiting the services provided to veterans under the RMFS.
In 2006, a similar AMA survey found that 59 per cent of specialists would continue to treat all veteran patients under the RMFS.
There was significant pressure on DVA funded health care at the time, with many examples of veterans being forced interstate to seek treatment or being put on to public hospital waiting lists.
The Government was forced to respond in late 2006 with a $600m funding package to increase fees paid under the RMFS and, while the AMA welcomed the package at the time, it warned that inadequate fee indexation would quickly erode its value and undermine access to care.
In this latest survey, this figure appears likely to fall to 43.8 per cent – underlining the AMA’s earlier warnings. The continuation of the indexation freeze puts a significant question mark over the future viability of the DVA funding arrangements and the continued access to quality specialist care for veterans.
The AMA continues to lobby strongly for the lifting of the indexation freeze across the Medicare Benefits Schedule and the RMFS, with these survey results provided to both DVA and the Health Minister’s offices. The Government promotes the DVA health care arrangements as providing eligible veterans with access to free high quality health care and, if it is to keep this promise to the veterans’ community, the AMA’s latest survey shows that it clearly needs to address this issue with some urgency.
Chris Johnson
Table 1
|
Which of the following statements best describes your response to the Government’s freeze on fees for specialists providing medical services to veterans under the Repatriation Medical Fee Schedule (RMFS): |
|
|
Answer Options |
Response Percent |
|
I am continuing to treat all veterans under the RMFS |
71.3% |
|
I am continuing to treat existing patients under the RMFS, but refuse to accept any more patients under the RMFS |
9.9% |
|
I am treating some veterans under the RMFS and the remainder either as fully private patients or public patients depending on an assessment of their circumstances |
10.8% |
|
I am providing some services to veterans under the RMFS (e.g. consultations) but not others (e.g. procedures) |
5.6% |
|
I no longer treat any veterans under the RMFS |
2.4% |
Table 2
|
Which of the following statements best describes your likely response if the Government continues its freeze on fees for specialists providing medical services to veterans under the RMFS: |
|
|
Answer Options |
Response Percent |
|
I will continue to treat all veterans under the RMFS |
43.8% |
|
I will continue to treat existing patients under the RMFS, but refuse to accept any more patients under the RMFS |
15.5% |
|
I will treat some veterans under the RMFS and the remainder either as fully private patients or public patients depending on an assessment of their circumstances |
21.1% |
|
I will provide some services to veterans under the RMFS (e.g. consultations) but not others (e.g. procedures) |
8.4% |
|
I will no longer treat any veterans under the RMFS |
11.2% |
[Comment] Total joint arthroplasty in younger patients: heading for trouble?
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are safe and effective surgical procedures for advanced degenerative osteoarthritis.1,2 Traditionally, the success of total hip and knee arthroplasty has been determined by the measurement of survival. In the Lancet, Lee Bayliss and colleagues3 now introduce the use of lifetime risk as a novel approach to illustrate the risk of revision surgery following joint replacement. Lifetime risk data, which describe the probability of an event occurring over the course of a lifetime, is useful to patients, clinicians, and other health-care professionals because it is easier to convey and understand than the commonly used survival rates.
Not alarmist, just the boring truth
DR JOHN ZORBAS, CHAIR, AMA COUNCIL OF DOCTORS IN TRAINING
The truth is often incredibly boring. It doesn’t sell papers. It doesn’t get people tuning in. It doesn’t win votes. And thus it follows that when things don’t make sense, one should assume incompetence before malice. But I’m finding it incredibly hard to suspend my disbelief when I stand back and take a look at the medical training system that we have in front of us today.
I’m not trying to be alarmist. I’m not here to tell you all that medical training is broken, and we should burn the books, burn the witches and behead Ned Stark. But I hope that I can convince you at the very least that the current progression to Fellowship is entirely unnatural and is fertile ground for unhealthy professional culture. To really understand this progression, I want you to pair up with each other, junior and senior doctors alike, and I want you to compare your respective paths through your medical journey. I find that often people have no idea what is or was on the other side of the fence. Let’s begin.
We finish medical school as the ultimate in medical pluripotency: the intern. We complete a year of heavily regulated and supervised training where we meander through medicine, surgery, emergency and whatever else might lie in our path that year. We then transition to residency, where without the pressure of training progression, we expand our medical buffet of specialisation and become more attuned to our final path in the journey. Armed with the knowledge of our experiences in areas such as general practice, ICU and plastics, and well rested from the safe hours worked, we apply for a training college. We get onto a program and begin to complete the pathway to specialty. Along the way, we have kids, and we do this by working part time at points along the way to balance the load. We complete our final exams and we become a Fellow of our chosen College, and apply for jobs in what is a reasonably well-balanced workplace. Right? Wrong. The truth is boring, but the truth is the truth, and this picture definitely isn’t the truth.
We finish medical school as the ultimate in medical pluripotency: the intern. We apply for internships, and a number of us will fail to get them as State governments are defaulting on their COAG agreement to provide medical graduates with internships. Without an internship, a number of doctors are unable to progress to general registration and are out before they begin. Those who remain become residents. With no national body to oversee PGY2+ terms, and with health services hungry to provide services to increasing populations with shrinking budgets, these residents work terms that don’t provide any meaningful experience. This veritable army of night cover and discharge summary monkeys are forced to scrounge around for the breadcrumbs falling off the training table. The smart ones quit, locum and complete further study, but not without further financial and temporal penalty. We’ve built a system in which the best way to advance your career is to quit the system for a while; a perverse incentive. This of course leaves behind fewer residents to fill the gaps in the roster, who are already at breaking point due to being denied leave for three years.
Nevertheless, you move towards a College. You identify the entry requirements and you undertake the extra mile to become a candidate with a chance. In some instances, that means completing a $5000 exam before you’re even a trainee. Once in, you work full-time and then the rest of it. You complete graduate diplomas, Masters and PhDs to progress. You fill your CV with publications and courses that cost thousands of dollars to progress. But you do it anyway. Because at this point you’re the blackjack player with a hard twelve. You’ve sunk enough cost into this game that you can’t quit, and there’s a glimmer of a nine sitting on top of that deck. But there are many more face cards, and maybe it’s just me, but I swear I’m seeing more and more doctors folding and busting around me.
So, you make it through. With everyone else. You’ve completed a number of extra qualifications and courses. With everyone else. You’ve participated in the medical arms race, and you’re surrounded by tens of thousands of other nuclear nations who’ll do anything for that job. The fat has been trimmed and now we’ve hit muscle. Welcome to exit block; a nation of Australian Fellows who can’t move on to consultant positions because we’re doing more with less, in every sense of the phrase. Competition is one thing, but when you’ve got multiples of trainees to every consultant position, you don’t have a competition. You’ve got a war.
I told you I wasn’t going to be alarmist and I stand by that. My examples above are all based on real life cases. I believe firmly in having a competitive workplace. I believe that smart hard work should be rewarded in the workplace. But this is not the system we currently have. We have a system that rewards the single-minded.
This is nobody’s fault. But it’s definitely our problem. It’s up to us as a profession to recognise that this isn’t about doctors eschewing hard work. It isn’t about people wanting an easy life. This is about a culture that has not kept up with the times and it’s important for those working in well-run institutions to recognise that this is not the norm anymore.
Latest submission to Senate on private health insurance
The following is an edited and condensed extract of the AMA’s submission to the ACCC report to the Senate on private health insurance.
In Australia, the public and private systems work together as a part of a health system that provides patients with universal access to affordable health care. The balance between the private and public system cannot be overlooked.
The private health sector is a large contributor to the system. In 2014-15, 42 per cent of all hospital separations were funded by private health insurance; where 50 per cent were public patients and the remainder were self-funded. Not only is it a large contribution, but it is a cost effective one. In 2014-15, there were 4.1 million privately insured hospital separations for approximately $12 billion in outlays, or around $3000 per separation, compared to 5.9 million separations in the public sector for a combined government outlay of $48.1 billion (or $8,100 per separation). While the service mix and complexity may differ between the sectors, the private sector very efficiently complements the public sector. If consumers withdraw from the private sector, these services will need to be provided by the public sector. Under current capacity, the public sector will either not meet the additional demand, or will only do so at a higher cost to governments.
We need to ensure that as private health insurers interact with patients and hospitals, the underpinning regulation promotes the efficient supply of health services. Private Health Insurance (PHI) has specific features that make the design of efficient regulation especially complex. This is further compounded by the specific historical development and place of PHI in the Australian context – as a form of supplementary insurance to Medicare, with the primary purpose of providing private hospital cover. Current regulation, as well as defining the scope of the cover PHI provides, includes restrictions on premiums through Community Rating and Lifetime Cover, means-tested subsidies for PHI take-up (the PHI rebate which is among the top 20 most expensive Federal Government programs), along with means tested tax penalties (the Medicare Levy Surcharge) for the failure to take out cover, and price controls over increases in PHI premiums.
Managed care
Australians can choose to obtain their health care solely from Medicare or use a combination of Medicare and PHI to meet their medical needs.
PHI offers several advantages over the public system: a patient has the option of being treated by their own doctor, they have more control over when and where they receive medical care, and the waiting times for elective surgery tend to be considerably shorter. In short, PHI provides choice for the patient and, without that choice, its value is diminished.
Yet there is a subtle, and defined, shift from a system of patient control to managed care occurring in Australia. Australians do not want US-style managed care imposed on a system that currently produces superior health outcomes at lower cost (USD$4420 here compared with $9451 in the United States). Managed care, in terms of health care, means a person agrees to only visit certain doctors and specialists within their health care plan – limiting their choice of practitioner. Australia and Australians have not had a public conversation about whether they agree to relinquish control over their health and their health system to the private health insurers. (This change has occurred through the change to the contracting with hospitals with no-pay clauses; publication of practitioner details; establishing closed shop referral databases; and demanding pre-approvals prior to surgery.)
Competition in the sector
The level of competition along the supply side of private health services impacts upon the competition between private health insurers. Both insurers and providers (hospitals and practitioners) have indicated that competition is not as effective as it might be.
Some of the inputs to the provision of health can be influenced or controlled by the private health insurer. These are generally limited to hospital contracts, but do stretch to the pre-approving of surgery. As a result, contracting between the insurers and hospitals (large groups through to day surgeries) has become more vexed and publicly acrimonious at times.
Contracting is a voluntary, deliberate, and legally binding agreement between two or more competent parties. However, it can be argued that firms are not operating in a competitive market and the factors at play are such that agreements are not voluntary. Hospitals need to have a contract with the major private health insurance funds (suppliers). Some insurers have such a strong market position that they would be considered price makers, where others are considered to be smaller, and thus price takers. Smaller insurers are beginning to contract as a collective to improve market power, and the Australian Health Service Alliance now represents 28 of the 36 registered health funds, creating what they claim is the third largest buying group.
Furthermore, a small day surgery that may be practitioner-led may not have an equal power relationship when entering a contracting arrangement, nor the ability to undertake the detailed financial modelling that insurers can use to gain a more attractive contracting outcome – this can effectively provide the ability for insurers to determine what small day practices remain viable. This is problematic as small day surgeries can remove cases from the higher cost environment of overnight hospitals, as well as be areas where innovation can flourish.
As a result of this imbalance, the AMA is beginning to see variations in contracts that shift the nexus of control from the provider/patient to the private health insurance fund – managed care.
Publication of a practitioner’s details
Private health insurers offer gap cover schemes to provide their members with certainty about out-of-pocket expenses for their privately insured medical care. Medical practitioners electing to participate in a gap cover scheme must agree to the terms and conditions that are set by the insurer. One of the common terms and conditions is that the medical practitioner agrees to information about them being published including their name, practice address, contact details, gap agreement usage and participation rate, and average gap charges.
Bupa has a ‘Find a Healthcare Provider’ section of its website. It provides information on the gap payment that may apply with providers it is contracted with, as well as information on the percentage of services, roughly, under which providers participate in its gap scheme.
Nib, Bupa and HBF are major shareholders of a system called Whitecoat, which owns a database that provides information on practitioner charging patterns using data gleamed from the HICAPS system. Under the Whitecoat system, a provider’s agreement with the payment processing system (HICAPS) will lead to publication in the directory. The directory is segmented by insurer, and only a customer of Nib can find Nib data about a practitioner’s billing practices or percentage of services provided under a no-gap or known gap scheme.
Not unlike Trip Advisor, the Whitecoat site also allows consumers to search, find and book a clinical provider as well as review and share their experience. Whitecoat has stated that the customer reviews are vetted to ensure they do not contain clinical details, however, members have raised concerns that the vetting process is not foolproof.
Already it hosts over 40,000 providers (thus far, mainly allied providers such as dentists) and shares 250,000 patient reviews. Around six million private health insurance members will have access to this information.
However, these types of websites have the potential for significant unintended consequences. Far from helping health consumers, posting outcomes of treatment online could lead to reduced access to care, particularly for patients with chronic and complex health problems.
Referrals databases for consumers and general practitioners
Medibank has announced it is providing information to the referrals database Healthshare that will allow general practitioners to identify specialists who charge gap fees.
This initiative will provide information to approximately 85 per cent of general practitioners as to which doctors are part of Medibank’s ‘no-gap’ or ‘known gap’ schemes. The converse of this is that general practitioners will therefore know which doctors are not part of Medibank’s ‘no-gap’ or ‘known gap’ schemes as they will not be on the Medibank list.
Effectively this action by Medibank (which will undoubtedly be followed by the other large funds) could have a detrimental impact upon the referrals received by practitioners who are not part of Medibank’s ‘no-gap’ or ‘known gap’ schemes, as patients are increasingly weighing gap charges into their decision on which specialist they choose. It is a closed shop, and it means that who is the most appropriate clinician for the referral, based on medical advice, may not be the consumer’s driving motivation.
Again, this is a private health insurer influencing the provision of services and determining who may provide a service and, since they set the ‘no-gap’ or ‘known gap’ amounts, at what price.
Setting of premiums
The regulation of PHI premiums sits on top of this complex regulatory environment. It has a dual goal of protecting consumers from excessive pricing and the Commonwealth from fiscal risk. However, it has been argued by the PHI sector that this regulation provides an inefficient outcome.
Many stakeholders are of the view that the compliance costs of the premium setting process are out of proportion to the benefits that are obtained. These concerns tended to focus on the process being too long, the amount of information required, and claims that outcomes would be the same even if the requests for increases in insurance were not reviewed.
A relevant consideration to the process and its ongoing appropriateness is whether market failures exist in the PHI market that justify the current suite of regulations. There are 36 PHI entities competing in the market. However, the Australian industry is highly concentrated. The two largest insurers, Medibank and Bupa, have 55.4 per cent of the market. The Private Health Insurance Administrative Council in 2013 indicated that there does not appear to be ‘unbridled competition’.
Premiums are a key driver in the choice of insurance for consumers. The increase in exclusionary products has not been at the expense of growth in excesses and co-payments that are also used to mitigate premium costs.This indicates that consumers are purchasing products with excesses, co-payments and exclusions to minimise their premiums. Therefore any methodology to set premiums must ensure that this product remains viable and attractive to consumers.
PHI used to be run mainly by not-for-profit funds. However, around 70 per cent of the insured population are now covered by ‘for-profit’ funds. The shift to a for-profit industry has created a greater need to ensure that there are sufficient profits to allow a respectable return to shareholders. It would appear that the private health insurers are not averse to increasing premiums in order to ensure a sufficient return for their shareholders. APRA data show an industry surplus (before tax) of $1.56 billion for the 2015-16 financial year (up from $1.45 billion for the previous year). Nib’s 2017 half-year results showed a sizable return on equity of 31.7 per cent.
The Federal Government has a decided stake in ensuring participation in PHI. The Government’s regulatory environment of incentives and penalties all but guarantees customers to private health insurers and has ensured that the PHI industry is one of the Australian economy’s more protected industries. However, it also has the effect of undermining consumer confidence in the product. Allowing an industry with limited competition to set its own premiums may contribute to a further decline in confidence.
The full submission can be found at: submission/ama-submission-accc-report-senate-private-health-insurance
Jodette Kotz
AMA Senior Policy Advisor
[Correspondence] Feedback of results to trial participants: be upfront or risk affront
We commend Erik Stenberg and colleagues for their well designed and delivered large randomised trial (April 2, p 1397),1 which investigated the utility of mesenteric defect closure in reducing the incidence of internal hernia after laparoscopic gastric bypass surgery. The study robustly answers the authors’ research question and a plenary audience at the European Obesity Summit in Gothenburg, Sweden, agreed almost unanimously that closure of mesenteric defects should now be standard practice.
[Comment] Volume expansion and contrast-induced acute kidney injury
There is an ever-increasing population at risk of being exposed to intravascular iodinated contrast because of the increasingly popular practice of imaging techniques in medicine and surgery. Despite efforts to improve the safety of these agents, there has been no fundamental improvement in contrast product development since the introduction of iso-osmolar contrast more than 20 years ago.1–3 Thus, clinicians have focused on strategies to decrease the risk of contrast-induced acute kidney injury by limiting contrast volume, giving adjuvant agents, and providing supportive care once the renal damage has occurred.

 more_vert
more_vert