In 1949, John Cade reported the use of lithium in the treatment of manic–depressive illness, thus ushering in the era of psychopharmacology.1 Clinicians soon observed that patients receiving long-term lithium therapy developed polyuria, suggesting that it might cause renal tubular damage.2 Many years later, evidence arose that it might also reduce renal function, although the initial report included some patients with lithium toxicity.3 A meta-analysis of 1172 patients across 14 studies found that 15% of patients had a reduced glomerular filtration rate (GFR).4 This led to a discussion of the lithium level sufficient to control symptoms but not to create permanent renal damage.5
Controversy regarding the level of risk persisted. One authority rejected the possibility of renal damage;6 however, a recent meta-analysis of 365 studies disagreed with this but concluded that the risk of renal replacement therapy (RRT) was low (18/3369 patients [0.5%]).7
The need for an epidemiological survey was advocated;8 however, until now, this has only been done within restricted geographical foci, with uncertain denominators associated with incidence rates. A study involving two Paris hospitals and a questionnaire sent to all nephrologists in France found that 0.22% of all RRT patients had received lithium treatment, without any other symptoms of lithium intoxication.9 A Swedish study of two regions found that 0.81% of RRT patients had kidney disease attributed to lithium-induced nephropathy (LiN), and 1.2% of lithium-treated patients had raised serum creatinine levels. These patients had consumed lithium for at least 12 years and their incidence of end-stage renal disease (ESRD) was sixfold greater than that of the general population.10 Another study found that patients receiving lithium for 15.6 years had a lower GFR compared with that of controls.11 The evidence suggests that ESRD occurs after long exposure to lithium, up to 23 years.10–12
Our study addresses the need for a nationwide survey of the epidemiology of ESRD associated with LiN. We investigated details of all patients commencing RRT within Australia with clinically diagnosed LiN. Analysis was performed on a de-identified data extract from the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA). Release of the data for this purpose was approved by the ANZDATA Executive.
We collected data from ANZDATA for all patients who commenced RRT with renal failure attributed to lithium toxicity between 1 January 1991 and 31 December 2011. We compared these patients with those who commenced RRT with renal failure due to other causes over the same period. We noted whether patients at the time of commencing RRT were smokers, whether diagnosis was via biopsy (available after 1 April 1997), and serum creatinine levels at time of entry (available after 1 April 1998). Estimated GFR was calculated using the four-variable Modification of Diet in Renal Disease formula.13 ANZDATA contains information about patients who receive chronic RRT (dialysis or transplantation) in Australia,14 and is noted for its completeness. We used Australian Bureau of Statistics information concerning number, age and sex distribution of the Australian population.15
We used Pearson χ2 and Mann–Whitney U tests to compare patient characteristics between groups. We compared biopsy rates and trends over time between LiN and other patients using logistic regression, with time by LiN interaction included. We directly standardised incidence rates for age and sex to the 1 June 2006 Australian population, with age in 10-year cohorts for patients aged 0–79 years, then a single cohort of patients aged ≥ 80 years. We used linear regression to investigate changes in age of commencing RRT, and Poisson regression to investigate the mean number of comorbidities.
No patients commenced RRT associated with LiN before 1991. However, between 1991 and 2011, 187 patients did so, compared with 38 316 patients who commenced RRT with renal disease attributed to other causes.
LiN patients were more likely than other patients to be women, to be white, to smoke and to have a higher body mass index, but were of similar age and less likely to have undergone renal biopsy (Box 1). Among 172 LiN patients with available data, 42 (22.4%) underwent a diagnostic kidney biopsy. Biopsy rates of LiN patients decreased over time (odds ratio per year, 0.74; 95% CI, 0.64–0.84; P < 0.001), which was more rapid than biopsy rates in non-LiN patients (P < 0.001 for the time by LiN interaction).
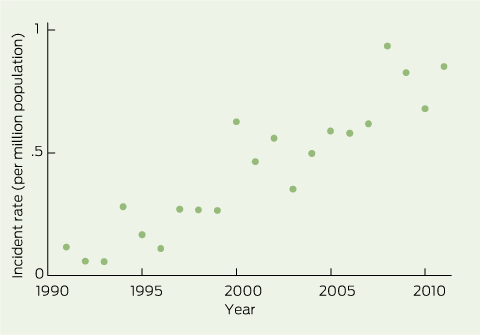
The number of patients commencing RRT associated with LiN increased over time, both in raw numbers, per population and as a proportion of all RRT patients. There were 0.14 cases per million population per year (95% CI, 0.06–0.22) in 1992–1996 and 0.78 (0.67–0.90) in 2007–2011. As a proportion of all incident RRT patients, LiN increased from 0.19% in 1992–1996 to 0.70% in 2007–2011 (Box 2). Age- and sex-standardised incidence rates were slightly lower than crude incidence rates for recent years (Box 2 and Box 3).
We found a marked increase in the incidence of clinically diagnosed LiN leading to RRT, rising from 0.14 cases per million population per year in 1992–1996 to 0.78 in 2007–2011. LiN accounted for 0.19% of all new RRT cases in 1992–1996, and 0.70% in 2007–2011. The former figure is close to the incidence reported in France in the late 1990s,9 while the latter is close to the incidence found in a recent Swedish study (0.81%).10 The similarity between standardised and crude incidence rates suggests that demographic changes do not explain the increase in the incidence of LiN.
Registry data have some limitations. Some cases of LiN may have been missed, especially early in the time series, when awareness of LiN may have been low. LiN is largely a clinical diagnosis, and most patients do not undergo biopsy. LiN patients commencing RRT were less likely to undergo biopsy, and rates are decreasing compared with those for other RRT patients. Diagnostic bias is possible with any registry data. Nephrologist awareness of irreversible LiN may have increased over time, with the publication of recent epidemiological studies10 and a systematic review.7 As such, the propensity to diagnose LiN as a cause of ESRD may have increased over time. Unknown confounders may also potentially increase rates of both bipolar disorder and kidney disease. Existing data do not allow us to determine numbers of patients with ESRD associated with LiN who are not recorded in ANZDATA, because they do not receive RRT. However, most people with ESRD in Australia do commence RRT.16 Data on the number of patients receiving long-term lithium treatment in Australia are not readily available.
We conclude that LiN is an uncommon cause of ESRD but is becoming more common. Many cases of LiN could be avoided through careful diagnosis of bipolar illness, restricted prescription of lithium, and careful follow-up of lithium serum levels and renal function. Improved record keeping, such as a lithium-monitoring book17 or electronic records, is important. We suggest that renal function (based on estimated GFR derived from serum creatinine levels, and proteinuria tests) and serum lithium levels should be monitored more frequently than 6 months, and certainly more than every 12 months, which some authors have suggested.18 This is most important for patients who have received lithium for many years. Clinicians should consider stopping lithium and using other suitable mood stabilisers (eg, sodium valproate19) if two consecutive readings suggest decreasing renal function, or if the estimated GFR is < 45 mL/min/1.73 m2.20
1 Characteristics of patients commencing renal replacement therapy (RRT) with lithium-induced nephropathy and all other patients in Australia, 1991–2011
|
Characteristic |
No. (%)* of patients with |
No. (%)* patients with other |
P |
||||||||||||
|
|
|||||||||||||||
|
Number of patients |
187 |
38 316 |
|
||||||||||||
|
Median age, years (IQR) |
60 (53–66) |
61 (47–71) |
0.99 |
||||||||||||
|
Men |
76 (40.6%) |
22 714 (59.3%) |
< 0.001 |
||||||||||||
|
Diabetes |
28 (15.0%) |
14 133 (36.9%) |
< 0.001 |
||||||||||||
|
Median body mass index, kg/m2 (IQR) |
28 (23–33) |
26 (22–30) |
< 0.001 |
||||||||||||
|
Smoker |
38 (20.3%) |
4749 (12.4%) |
0.001 |
||||||||||||
|
Median eGFR, mL/min/1.73 m2 (IQR) |
7.2 (5.4–9.9) |
7.2 (5.3–9.7) |
0.78 |
||||||||||||
|
European ancestry |
183 (97.9%) |
30 733 (80.2%) |
< 0.001 |
||||||||||||
|
Biopsy† |
42 (22.4%) |
10 079 (26.3%) |
|
||||||||||||
|
|
|||||||||||||||
|
eGFR = estimated glomerular filtration rate, calculated using four-variable Modification of Diet in Renal Disease formula;13 only available for patients who commenced RRT after 1 April 1998. IQR = interquartile range. * Unless otherwise indicated. † Not available before 1997. |
|||||||||||||||
2 Number of patients commencing renal replacement therapy (RRT), 1987–2011
|
Years |
No. of patients receiving |
Crude incidence rate/year/million population (95% CI) |
Standardised incidence |
Proportion of all incident RRT patients (95% CI) |
|||||||||||
|
|
|||||||||||||||
|
1987–1991 |
2† |
0.02 (0–0.07) |
0.02 (0.00–0.07) |
0.20% (0–0.49%) |
|||||||||||
|
1992–1996 |
12 |
0.14 (0.06–0.22) |
0.13 (0.05–0.21) |
0.19% (0.08%–0.30%) |
|||||||||||
|
1997–2001 |
36 |
0.36 (0.20–0.52) |
0.32 (0.18–0.47) |
0.40% (0.27%–0.53%) |
|||||||||||
|
2002–2006 |
52 |
0.51 (0.42–0.59) |
0.43 (0.36–0.51) |
0.48% (0.35%–0.62%) |
|||||||||||
|
2007–2011 |
85 |
0.78 (0.67–0.90) |
0.65 (0.54–0.76) |
0.70% (0.55%–0.85%) |
|||||||||||
|
|
|||||||||||||||
|
* Standardised incidence rates were directly age- and sex-standardised to the 1991 populations. † Both patients in 1991. |
|||||||||||||||

 more_vert
more_vert