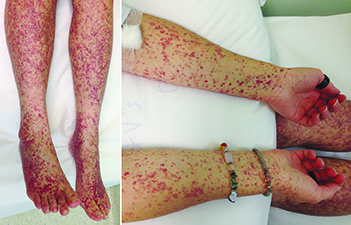
We describe a case of Henoch–Schönlein purpura (HSP) in a 19-year-old woman triggered by a diarrhoeal illness from Yersinia enterocolitica. The patient subsequently developed a widespread purpuric rash on the upper and lower limbs (Box), spasmodic abdominal pain, polyarthralgia and macroscopic haematuria. A skin biopsy confirmed leukocytoclastic vasculitis. Immunofluorescence testing on a percutaneous renal biopsy revealed strong mesangial staining for IgA. While post-infectious proliferative glomerulonephritis with Y. enterocolitica is described in several case series, the syndrome of HSP after this infection has rarely been reported.1,2 This case reinforces that any immune stimulus in IgA-producing tissue, including gastrointestinal infection, can trigger HSP.
Preference: Renal Medicine
1029
Reversal of end-stage renal failure using direct-acting antiviral agents for chronic hepatitis C
Clinical record
A 58-year-old Eritrean woman presented to hospital in December 2014 with a vasculitic rash, peripheral oedema, acute renal impairment and microscopic haematuria with proteinuria. She had a history of genotype 4 hepatitis C virus (HCV) infection, which was unsuccessfully treated with pegylated interferon and ribavirin in 2010. Results of laboratory investigations showed a pattern consistent with mixed cryoglobulinaemia (Box 1). After a renal biopsy to assess progressive renal impairment, a diagnosis of membranoproliferative glomerulonephritis (MPGN) was made. The patient began taking an angiotensin-converting enzyme inhibitor and diuretic therapy.
The patient’s creatinine level continued to worsen, from 202 μmol/L (reference interval [RI], 45–90 μmol/L) (estimated glomerular filtration rate [eGFR], 27 mL/min/1.73 m2 [RI, > 60 mL/min/1.73 m2]) in January 2015 to 412 μmol/L (eGFR, 10 mL/min/1.73 m2) in July 2015. The renal impairment was associated with increasing serum cryoglobulin levels, worsening proteinuria and increased requirement for ascitic drainage. The ascitic fluid was a transudate, and a transjugular liver biopsy excluded cirrhosis. Infection and malignancy were also excluded. As the eGFR was below 15 mL/min/1.73 m2 and fluid overload had become refractory to medical management, preparations for dialysis were made and a Tenckhoff (peritoneal dialysis) catheter was inserted. At this stage, direct-acting antiviral medication was obtained through a compassionate access program to treat the HCV infection.
The patient began a 12-week course of HCV treatment with paritaprevir–ritonavir (150 mg/100 mg) and ombitasvir (25 mg) plus ribavirin (200 mg) daily on 2 July 2015. Ribavirin was discontinued at Week 5, as her haemoglobin level had fallen from a baseline of 102 g/L (RI, 115–160 g/L) to 76 g/L. Overall, the treatment was well tolerated. HCV viral load was undetectable at Week 2, and sustained viral response (SVR) 12 weeks after completion of treatment was achieved, indicating the HCV was cured. Creatinine levels improved markedly with treatment, associated with a rapid decline in serum cryoglobulin level and improvement in the urine albumin:creatinine ratio (Box 2). The ascites was last drained on 24 August 2015, and the Tenckhoff catheter was subsequently removed.
Mixed cryoglobulinaemia is a frequently encountered extrahepatic complication of chronic HCV infection and can be associated with considerable morbidity and mortality.1 The most common renal manifestation of mixed cryoglobulinaemia is MPGN, which is associated with a mixed cryoprecipitate, hypocomplementaemia, positive rheumatoid factor, microscopic haematuria and proteinuria.1 The clinical presentation ranges from asymptomatic proteinuria to nephritic or nephrotic syndrome.1 Progression to end-stage renal failure occurs in 10–33% of patients.1
Treatment of mixed cryoglobulinaemia due to HCV is reserved for symptomatic patients and centres on viral eradication.2 In a series of 86 patients with HCV infection and cryoglobulinaemic vasculitis treated with pegylated interferon and ribavirin, remission of symptoms occurred in 88% of patients who achieved SVR.2 However, management of patients with concurrent renal disease is complicated by the difficulties associated with using pegylated interferon and ribavirin in the setting of severe renal failure, and a lower response rate is reported in this cohort.2 In clinical practice, interferon and ribavirin therapy is therefore rarely pursued in patients receiving dialysis. In addition, the reversibility of renal impairment in the setting of HCV-induced cryoglobulinaemia has traditionally been thought to be poor, particularly once end-stage renal disease has been reached.
There are limited data available on the outcomes of interferon-free HCV treatment regimens in patients with mixed cryoglobulinaemia.3 The efficacy of sofosbuvir-based regimens has been studied in a series of 12 patients with HCV-associated mixed cryoglobulinaemia, with seven patients also having renal impairment related to MPGN.3 In this study, patients who achieved SVR had decreased proteinuria and improved renal function; however, the degree of baseline renal impairment was mild (eGFR, 30–60 mL/min/1.73 m2).3 Sofosbuvir is not currently recommended for use in patients with an eGFR < 30 mL/min/1.73 m2.3
The combination of ombitasvir and ritonavir-boosted paritaprevir is an interferon-free antiviral regimen for the treatment of genotype 4 HCV infection, with the addition of dasabuvir required for genotype 1 HCV infection.4 The efficacy and safety of this combination in patients with end-stage renal disease have been demonstrated in a pilot trial of 20 patients with genotype 1 HCV infection,5 although the impact of viral clearance on renal function has not been reported.
A literature review of the PubMed database on this topic did not identify other cases, so we believe this to be the first reported case of significant recovery of renal function in this clinical setting.
Lessons from practice
-
In patients with chronic hepatitis C virus (HCV) infection and renal failure from cryoglobulin-related glomerulonephritis, direct-acting antiviral medications may be safe and effective, and successful treatment may significantly improve renal function.
-
Chronic HCV infection can have numerous extrahepatic manifestations that may abate considerably after virus eradication.
-
Patients with chronic HCV infection who were previously considered difficult to treat due to medical comorbidities should be reassessed for HCV treatment with direct-acting antiviral medications.
Box 1 –
Results of diagnostic investigations
|
Laboratory test |
Result |
Reference interval |
|||||||||||||
|
|
|||||||||||||||
|
Urinary protein |
+++ |
nd |
|||||||||||||
|
Urinary blood |
+++ |
nd |
|||||||||||||
|
Albumin:creatinine ratio (mg/mmol) |
121.1 |
< 3.5 |
|||||||||||||
|
Creatinine (μmol/L) |
179 |
45–90 |
|||||||||||||
|
Cryoglobulins (g/L) |
0.44 |
< 0.08 |
|||||||||||||
|
C3 (g/L) |
0.80 |
0.88–1.98 |
|||||||||||||
|
C4 (g/L) |
0.04 |
0.16–0.52 |
|||||||||||||
|
Rheumatoid factor (kU/L) |
117 |
< 14 |
|||||||||||||
|
Hepatic venous pressure gradient (mmHg) |
10 |
1–5 |
|||||||||||||
|
|
|||||||||||||||
|
nd = not detectable. |
|||||||||||||||
Box 2 –
Results of investigations showing response to treatment with direct-acting antiviral medications
|
Laboratory test |
Reference interval |
Result |
|||||||||||||
|
Before treatment (July 2015) |
End of treatment (Sept 2015) |
12 weeks after treatment (Dec 2015) |
|||||||||||||
|
|
|||||||||||||||
|
Haemoglobin (g/L) |
115–160 |
102 |
125 |
113 |
|||||||||||
|
Albumin (g/L) |
35–50 |
33 |
35 |
40 |
|||||||||||
|
Creatinine (μmol/L) |
45–90 |
412 |
186 |
196 |
|||||||||||
|
eGFR (mL/min/1.73 m2) |
> 60 |
10 |
25 |
24 |
|||||||||||
|
HCV viral load (IU/mL) |
nd |
2.00 × 106 |
nd |
nd |
|||||||||||
|
Cryoglobulins (g/L) |
< 0.08 |
3.46 |
0.84 |
0.56 |
|||||||||||
|
Albumin:creatinine ratio (mg/mmol) |
< 3.5 |
394 |
nr |
67.7 |
|||||||||||
|
C3 (g/L) |
0.88–1.98 |
0.67 |
0.94 |
0.87 |
|||||||||||
|
C4 (g/L) |
0.16–0.52 |
< 0.03 |
0.06 |
0.06 |
|||||||||||
|
|
|||||||||||||||
|
eGFR = estimated glomerular filtration rate. HCV = hepatitis C virus. nd = not detectable. nr = no result available. |
|||||||||||||||
The inequitable burden of group A streptococcal diseases in Indigenous Australians
We need to fill evidence gaps and make clinical advances to reduce these diseases of disadvantage
Group A streptococcal (GAS) infections contribute to the excess burden of ill-health in Indigenous Australians, causing superficial infection, invasive disease, and the autoimmune sequelae of acute rheumatic fever (ARF) and acute post-streptococcal glomerulonephritis (APSGN) (Box 1).1–6 GAS diseases declined in the broader Australian population during the 20th century, largely as a result of improved living conditions,7 but this is not the case in Indigenous Australians. GAS infections and their sequelae persist at unacceptably high rates in remote Australia, on par with or higher than those in low income settings internationally.8 GAS infections globally represent social disadvantage.5,8 Poverty, household overcrowding and distance from health care services are the main drivers.9
GAS impetigo
In remote Australian communities, impetigo, predominantly caused by GAS infection,2,10,11 affects a median of 45% of Indigenous children at any one time.3 This high prevalence is testament to the poor environmental conditions9 and household overcrowding in Indigenous communities.10,12 A high burden of circulating group A streptococcus strains13 and scabies are contributory factors.2 Further, skin infections are also “normalised”, which contributes to the burden as it is not seen as a significant problem — affecting both health care-seeking behaviour14 and the response by clinicians when patients present with other complaints.15 Despite being under-recognised, GAS impetigo is of public health importance. Untreated, it can lead to APSGN, with resultant acute cardiac morbidity from hypertension.1 Although acute case fatality rates are low (< 2%),1 APSGN in childhood increases the risk of chronic kidney disease later in life in Indigenous Australians.16
Precursor to rheumatic fever
ARF and subsequent rheumatic heart disease (RHD) are the most severe and life-threatening post-streptococcal diseases. Mortality rates from RHD in Indigenous Australians are the highest reported in the world.1 Traditionally, GAS pharyngitis has been considered the lone antecedent to ARF.17 Yet, in remote tropical Australia, GAS pharyngitis is uncommonly reported and GAS skin infections are hyper-endemic.12 Thus, impetigo, rather than pharyngitis, may be the driver. The findings of studies to clarify this dilemma have not been definitive.6,12 Recently, a New Zealand molecular epidemiological study using M-protein (emm) cluster typing found that 49% of ARF-associated GAS strains from isolates were emm pattern D (skin pattern) types.18 Further studies examining the causal link between GAS impetigo and RHD remain a priority if we are to make further progress towards the primary prevention of RHD.12
Current approaches to GAS infection control
Community and primary health programs
For decades, the focus in the Northern Territory has been on control of skin disease,10,11,19 although treatment for sore throat is also promoted.20 Community skin days and mass drug administration with permethrin11 have been successful, but their impact is not sustained. More recently, a better tolerated treatment regimen for impetigo was reported, with oral co-trimoxazole proven to be non-inferior to intramuscular penicillin;10 and mass drug administration with oral ivermectin shown to be an effective population approach to reducing scabies and impetigo.19 However, to date, no approach in Australia has achieved a sustained reduction in GAS impetigo. Overcrowding and population mobility are among the contributing factors and, more recently, the contribution of community members with crusted scabies as core transmitters of the scabies mite has been recognised.19 New approaches to management of crusted scabies in the NT include surveillance under public health legislation21 and coordinated case management.22 However, there remains a need to target the other contributing factors, particularly overcrowding, before sustained reductions can be achieved.
Policy and legislation
The only GAS diseases that have any jurisdiction-level policies or strategies are skin infections, APSGN, ARF and RHD. The NT has well established, evidence-based guidelines for community-level skin sore and scabies control, and an APSGN outbreak response.23 Other jurisdictions have adopted the APSGN guidelines when needed, but do not have legislation requiring notification of the disease. Through the national Rheumatic Fever Strategy, the Australian Government has funded the development and maintenance of register-based RHD control programs for monitoring the RHD burden and coordination of care, with a focus on secondary prophylaxis, in the NT, Queensland, Western Australia and South Australia, as well as the establishment of the National Coordination Unit.24,25 New South Wales established a statewide register in 2015.26 Centralised coordination of secondary prophylaxis, the only cost-effective method proven for RHD control,27 through electronic registers is advantageous for mobile populations if the systems are shared and accessible to all health service providers. Given that RHD has the highest differential mortality between Indigenous and non-Indigenous Australians of any preventable condition,28 continuation of Rheumatic Fever Strategy funding is essential if Australia is to achieve its Closing the Gap targets.
Areas for future focus to close the gap in GAS infection outcomes
Heightened surveillance
Currently, no GAS diseases are nationally notifiable,29 but a number are notifiable in different jurisdictions (Box 2). Passive surveillance via notifiable disease reporting would be the cheapest and least resource-intensive method30 for monitoring GAS diseases and their sequelae in remote Australia. ARF, scarlet fever, and puerperal fever were all nationally notifiable in Australia before 1990.31 All three are no longer nationally notifiable.
Surveillance programs for APSGN, ARF and invasive GAS infection in the NT or for RHD in WA, SA and NSW could be replicated elsewhere. In New Zealand, diseases that disproportionately affect Maori and Pacific Islander peoples are prioritised; national notification of ARF is legislated,32 and there are well resourced school screening programs for sore throat and skin infection.33 Legislating for notification of GAS diseases that disproportionately affect Australian Indigenous people would facilitate accurate disease monitoring and directed public health response, and provide advocacy tools for Indigenous health campaigners to demand action.
Primary prevention
Future approaches to comprehensive skin disease control programs will incorporate sustainable community-wide approaches, acceptable clinical treatments, appropriate contact management, evidence-based prevention and community control initiatives that are embedded in primary health care. Earlier skin disease control programs were effective initially,11 but were unsustainable due to the cost of using a largely external workforce. Combining streamlined treatment guidelines for impetigo, scabies and crusted scabies into training, health promotion and environmental health activities that are culturally secure will be critical. The role of skin disease control in ARF prevention is unclear, and requires a better understanding of the relationship between GAS impetigo and ARF. Monitoring the impact of sustained impetigo control measures on the incidence of ARF could be included in skin control programs to help us understand the potential role for impetigo control as a primary prevention strategy for ARF.
Research and development of new technologies
Development of a GAS vaccine
A vaccine against group A streptococcus would be a major advance in reducing the excess burden of GAS disease in Indigenous Australians, particularly in the current absence of a cost-effective primary prevention strategy for ARF. Several M-protein-based vaccines have progressed to human clinical trials,34 but none have yet moved beyond phase II trials. The need to cover multiple diverse strains and a standardised immunoassay for efficacy and immunogenicity monitoring are current barriers to vaccine development.35 The Coalition to Accelerate New Vaccines for group A Streptococcus (CANVAS), a joint initiative between the Australian and New Zealand governments, is tackling these barriers to advance GAS vaccine research.18
Long-acting penicillins for secondary prevention of ARF
The mainstay of secondary prevention of ARF remains intramuscular injections of benzathine penicillin every 28 days for a minimum of 10 years.36 A longer-acting, less painful way of administering penicillin would overcome some of the avoidance and acceptability issues with the current formulation.37 Key questions remain before a better alternative can be delivered, but progress is underway36 through studies examining the pharmacokinetics, patient preferences and the rationale behind the current formulation.
Primordial prevention
Although there is progress towards a potential vaccine and longer-acting antibiotics, these remain distant possibilities. Moreover, the large reductions in ARF and APSGN occurred in the wider population without these technologies.7 Indigenous people have not benefited from improvements in the social determinants of health that resulted in the virtual elimination of these conditions in the non-Indigenous population. As a contribution to improving socio-economic disadvantage, clinicians can provide health data to help quantify the disadvantage that exists. Capacity building through support and training of Indigenous clinicians is a necessity for providing accessible primary health care. Further capacity building will see Indigenous health practitioners become leaders in policy and research to facilitate Indigenous community control over health programs and funding. Empowering the community to vanquish the effects of more than two centuries of colonisation, racism and oppression should be at the forefront of policy development if we are to achieve equity in the social determinants of health and reduce the prevalence of diseases that represent disadvantage, including GAS infections and their sequelae.
Conclusions
Given the ongoing mortality and morbidity from chronic kidney and heart disease due to GAS infection in Indigenous Australians, we must address more effectively the treatment and prevention of the precursors, GAS impetigo and pharyngitis. An essential step in improved prevention and control is effective surveillance of GAS conditions. Quality surveillance data would quantify the disease burden at both a jurisdictional and national level, providing important information to guide resource allocation. Effective, sustainable skin disease control programs embedded within the activities of the existing workforce are another priority. New prevention initiatives in GAS vaccines and longer-acting penicillin therapy are progressing. However, despite these clinical advances, the top priority remains the need to improve the quality of housing and access to health care that continue to disadvantage remotely living Indigenous Australians — these are the underlying reasons for the inequity in GAS outcomes that continue today.
Box 1 –
The global and local burden of group A streptococcal (GAS) skin infections and pharyngitis and their sequelae
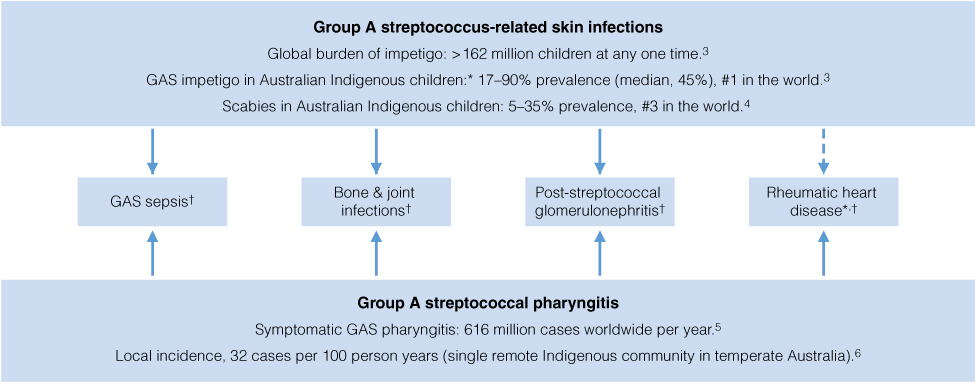
* Indigenous Australian children have the highest reported burden in the world.3,5 † Incidence in Indigenous children surpasses that in non-Indigenous children.1,5
Box 2 –
Diseases caused by group A streptococcal infections that are notifiable under state and territory public health legislation in each state or territory of Australia29
|
Notifiable group A streptococcus-related condition |
Australian state or territory |
||||||||||||||
|
ACT |
NSW |
NT |
Qld |
SA |
Tas |
Vic |
WA |
||||||||
|
|
|||||||||||||||
|
Acute post-streptococcal glomerulonephritis |
|
|
Yes |
|
|
|
|
|
|||||||
|
Acute rheumatic fever |
|
Yes |
Yes |
Yes |
Yes |
|
|
Yes |
|||||||
|
Invasive group A streptococcal infection |
|
|
Yes |
Yes |
|
|
|
|
|||||||
|
Rheumatic heart disease |
|
Yes* |
|
|
Yes |
|
|
Yes |
|||||||
|
Scarlet fever |
|
|
|
|
|
|
|
Yes |
|||||||
|
|
|||||||||||||||
|
ACT = Australian Capital Territory. NSW = New South Wales. NT = Northern Territory. Qld = Queensland. SA = South Australia. Tas = Tasmania. Vic = Victoria. WA = Western Australia. * Notifiable in people aged under 35 years. |
|||||||||||||||
A case of acute phosphate nephropathy
A 74-year-old woman presented with a 2-day history of abdominal pain and nausea, 1 week after undergoing upper and lower endoscopies. Her comorbidities included hypertension, dyslipidaemia, sigmoid diverticulosis, transient ischaemic attack, peripheral vascular disease and mild valvular heart disease.
Medications taken before admission included telmisartan–hydrochlorothiazide, sustained-release verapamil, prazosin, rosuvastatin and aspirin.
Admission blood tests indicated acute kidney injury, with a serum creatinine concentration of 218 μmol/L (reference interval [RI], 46–99 μmol/L) and an estimated glomerular filtration rate of 19 mL/min/1.73 m2 (RI, > 60 mL/min/1.73 m2); her creatinine level 7 months earlier was 69 μmol/L. She was also hypokalaemic (potassium level, 2.9 mmol/L [RI, 3.5–5.1 mmol/L]), hypocalcaemic (corrected calcium concentration, 1.97 mmol/L [RI, 2.15–2.6 mmol/L]) and uraemic (urea concentration, 14.4 mmol/L [RI, 2.9–8.2 mmol/L]). However, she was normophosphataemic (phosphate level, 1.14 mmol/L [RI, 0.81–1.45 mmol/L]). Her urinary sediment was relatively benign, with a protein-to-creatinine ratio of 17 g/mol (RI, < 15 g/mol), an albumin-to-creatinine ratio of 3.4 g/mol (RI, < 1 g/mol) and no red cells present. Renal ultrasound showed normal sized kidneys with no evidence of obstruction.
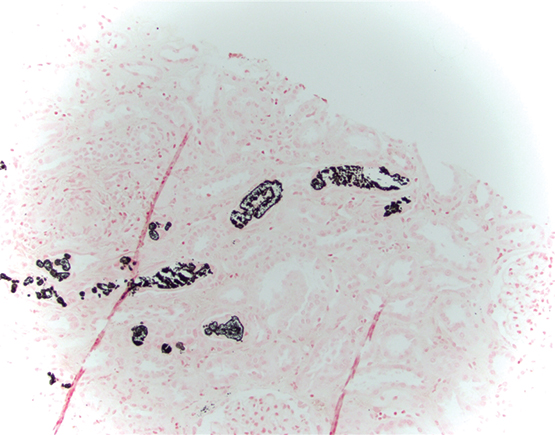
A medication review revealed she had taken 48 g of oral sodium phosphate before undergoing colonoscopy. Given the lack of improvement in renal function following intravenous hydration and withholding telmisartan–hydrochlorothiazide, a renal biopsy was performed. Light microscopy demonstrated non-polarising calcifications within the tubular cells and interstitium consistent with acute phosphate nephropathy (APN). A von Kossa stain confirmed calcium phosphate crystals (Box).
Our patient’s serum creatinine level stabilised at 130–140 μmol/L before discharge. A review in the outpatient’s clinic 1 month after discharge showed no further improvement in serum creatinine levels (131 μmol/L).
The incidence of APN is underestimated because many cases are clinically silent and can occur without evidence of hyperphosphataemia.1,2 APN occurs due to a combination of hypovolaemia and a sudden increase in serum phosphate concentration. Hypovolaemia induces proximal tubule salt and water reabsorption, resulting in a large phosphate load to the distal nephron and subsequent precipitation of calcium phosphate in the distal tubule and collecting duct.3
Associated risk factors for developing APN include increasing age, female sex, hypertension, diabetes, degree of hyperphosphatemia, pre-existing chronic kidney disease, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, diuretics, lithium and non-steroidal anti-inflammatory drugs.1,2
Patients who develop APN have a high probability of progressing to chronic kidney disease.1 Markowitz and colleagues studied a cohort of 21 patients with biopsy-proven APN. The mean serum creatinine level 17 months after oral sodium phosphate ingestion was 240 μmol/L. Four patients progressed to end-stage renal failure, requiring dialysis at the mean follow-up of 16.7 months.1 In an Icelandic study, the baseline serum creatinine level was 82 μmol/L in 15 patients before their diagnosis of APN. The mean serum creatinine level at 4-month follow-up was 180 μmol/L, with one patient becoming dialysis dependent.4
APN is an important adverse event to consider when using oral sodium phosphate for bowel preparation in at-risk populations. The consequences of APN may be significant and long term, as there is increased risk of progression to chronic kidney disease and end-stage renal failure. Thus, the clinician needs to demonstrate heightened pharmacovigilance when prescribing such agents and an increased clinical suspicion in diagnosing this condition.
A multidisciplinary renal genetics clinic improves patient diagnosis
Developments in genomic science are disproportionately in advance of their translational clinical application. Multidisciplinary clinics are proposed to overcome this1 in many medical fields.2 This is especially so in nephrology, which is typified by significant community disease burden3 and heritability.4 Several renal genetics clinics (RGCs) operate overseas, although their models and outcomes are largely unreported. The first multidisciplinary RGC in Australasia commenced at the Royal Brisbane and Women’s Hospital in August 2013, involving a clinical geneticist, nephrologist, genetic counsellor, and ancillary clinical and diagnostic services. The departments of clinical genetics and nephrology jointly operate the RGC. The clinical geneticist and nephrologist see families in the same appointment, maximising use of time. In this article, we report this clinical service’s initial outcomes and model for mainstreaming genetic medicine.
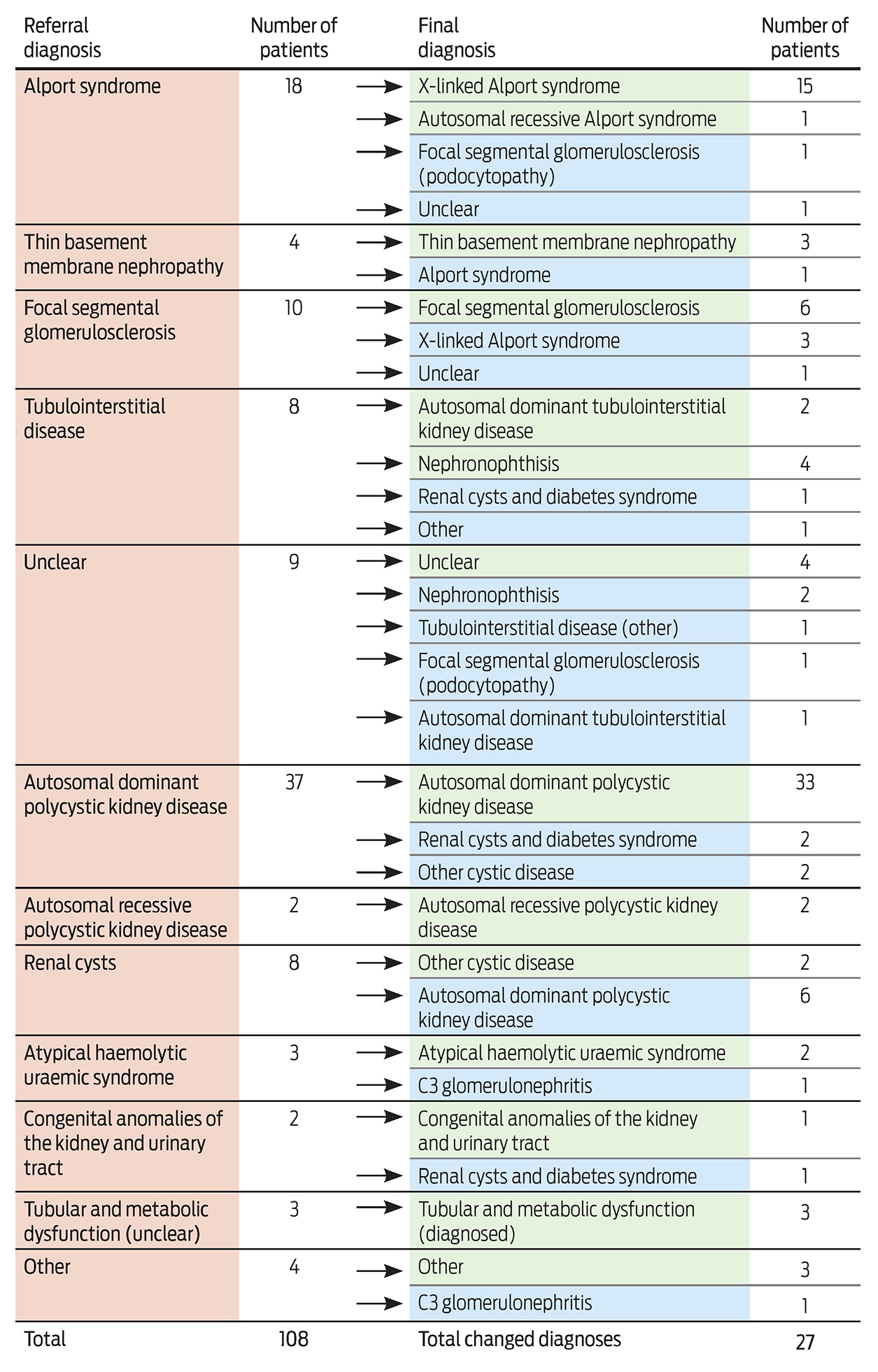
We undertook a retrospective cohort study of patients who attended the Royal Brisbane and Women’s Hospital Renal Genetics Clinic during its first 2 years of operation (1 August 2013 to 31 August 2015; ethics approval reference, HREC/14/QRBW/187). During this period, 108 patients from 100 families were seen; the median age was 41 years (range, 13–86 years). Most patients were referred by a public or private sector nephrologist (47% [51/108] and 28% [30/108], respectively), and 81% (87/108) had an existing genetic renal diagnosis, 45% (49/108) had extra-renal clinical features and 65% (70/108) had a family history of renal disease. Existing renal diagnoses were diverse, and the most common were autosomal dominant polycystic kidney disease (34% [37/108]), Alport syndrome (17% [18/108]) and focal segmental glomerulosclerosis (7% [8/108]).
The overlapping reasons for referral were for a diagnosis (67% [72/108]), a discussion about a diagnosis (27% [29/108]) and genetic counselling (81% [87/108]). Clinical and family histories and results of clinical investigations were reviewed. Differential diagnoses were discussed for 68% of patients (73/108), disease information was provided to 89% (96/108), and genetic counselling provided to 67% (72/108).
Genetic testing was ordered for 69% of patients (75/108). Of a total of 83 tests, results were positive for 39% (32/83), negative for 30% (25/83), “variant of uncertain significance” for 7% (6/83) and pending for 24% (20/83). Negative genetic test results have enabled 12 of the families to enrol in a research study.5 To date, the clinical diagnosis has changed for 27 of the 108 patients (25%) (Box), enabling correct diagnosis, accurate genetic counselling, identification of at-risk individuals, access to assisted reproductive technologies and altered medical management.
This RGC model is novel in Australasia and its results are among the first to be reported internationally. In its first 2 years of operation, patients underwent clinical appraisal and a tailored combination of differential diagnosis discussion, disease information provision and genetic counselling. Genetic testing was often, but not always, used, with results confirming or clarifying a diagnosis for about half of the patients. Overall, the diagnosis was changed in a quarter of patients. This clinic model is inclusive, flexible and multidisciplinary while demonstrably improving patient diagnosis and care. We believe that it is a viable, translational and patient-focused clinical template for effective introduction of genetics and genomics into everyday clinical practice.
Change treatment for small tumours: expert
A leading UK cancer expert says Australian patients would benefit from a change in the way small tumours are treated.
Professor Andreas Adam from King’s College London is in Australia for the Clinical Oncology Society of Australia’s Annual Scientific Meeting in Hobart this week.
He says treatment of small tumours using minimally invasive techniques with medical imaging helps avoid invasive surgery and longer hospital stays.
Interventional oncology is particularly important in the treatment of kidney cancers.
“Typically, smaller tumours in the kidney have been monitored by urologists rather than operated on. The risks of surgery for these patients have been considered not to be justified, because they rarely give rise to secondary tumours,” he says.
“However, there is increasing evidence from large studies that small kidney tumours give rise to secondaries more often than was previously thought. This means we need to be more proactive in assessing and treating this type of cancer.
Related: Skin rash, a kidney mass and a family mystery dating back to World War II
“Interventional radiologists can destroy these tumours using techniques that have lower risks than surgery, thus justifying treatment at an earlier stage rather than waiting for them to grow.”
These learnings could be applied in Australia, Clinical Oncology Society of Australia President, Professor Mei Krishnasamy says.
“Interventional radiation oncology is an emerging field in Australia and the shortage of practitioners could be having a negative impact on our cancer patients,” Professor Krishnasamy says.
“Sharing knowledge about the benefits of this innovative form of treatment is crucial to ensuring that interventional oncology and radiology gets the recognition it deserves.”
The theme of this year’s COSA ASM is ‘Rare cancers – Common goals’.
Latest news:
Legionella pneumonia with severe rhabdomyolysis
A 41-year-old man presented with a 5-day history of fever, non-productive cough and shortness of breath associated with copious watery diarrhoea and excessive fatigue. Apart from heavy smoking and alcohol misuse, there was no significant past medical history including no recent travel.
On examination, we found the patient to be diaphoretic, tachycardic (144 beats/min) and tachypnoeic (42 breaths/min), with a temperature of 39.7°C and blood pressure of 155/105 mmHg. He had an oxygen saturation of 98% after 8 L/min of supplemental oxygen. Auscultation of the chest elicited coarse crepitations over the right lung base and normal heart sounds. He had generalised weakness but no focal neurological deficit.
Laboratory results on admission showed a sodium level of 126 mmol/L (reference interval [RI], 137–145 mmol/L); chloride level, 96 mmol/L (RI, 100–109 mmol/L); potassium level, 3.3 mmol/L (RI, 3.5–4.9 mmol/L); urea level, 10.7 mmol/L (RI, 2.7–8.0 mmol/L), rising to a peak of 46.5 mmol/L; and creatinine concentration 310 μmol/L (RI, 50–120 μmol/L), rising to a peak of 908 μmol/L. Liver function test results were moderately abnormal: aspartate transaminase, 471 U/L (RI, < 45 U/L; peak, 860 U/L); alanine transaminase, 200 U/L (RI, < 55 U/L; peak, 1309 U/L); and lactate dehydrogenase, 4379 U/L (RI, 110–230 U/L). There was leucocytosis, with a white blood cell count of 13.6 × 109/L (RI, 4.0–11.0 × 109/L), neutrophilia (87.1%), mild thrombocytopenia (platelet count, 114 × 109/L (RI, 150–400 × 109/L) and an elevated C-reactive protein level (360 mg/L, RI, < 10 mg/L).
The patient’s serum creatine kinase (CK) level was elevated to 89 860 U/L (RI, < 250 U/L) and his serum myoglobin level was 42 268 μg/L (RI, < 85 μg/L). CK levels peaked at 141 116 U/L on Day 3.
On admission, the patient’s chest x-ray showed right middle lobe consolidation. His renal ultrasound was unremarkable. Subsequently, a sputum culture grew Legionella pneumophila serogroup 1. Urinary antigen test results were also positive for this organism, further confirmed by serology (antibody titre of 1 : 128).
The patient was admitted to the intensive care unit and treatment commenced with ceftriaxone 2 g (stopped after serological confirmation of Legionella) and azithromycin 500 mg daily (continued for 14 days). He was oligoanuric and required haemodiafiltration, which improved urine output along with renal functions. The principal cause for his renal failure was postulated to be severe rhabdomyolysis. Although dehydration related to diarrhoea may have been contributory, it was not deemed to be the main cause of renal failure since the patient was never hypotensive during admission. He was discharged from hospital after 20 days, with normal electrolyte levels and near-normal renal function.
Postinfection rhabdomyolysis is a rare complication associated with Legionella, first described in 1980.1 The mechanism of rhabdomyolysis associated with Legionella infection is unknown. Theories include direct invasion of Legionella into the muscle itself, or release of its endotoxin into the circulation with subsequent muscle injury.2
A literature review identified only 23 cases of rhabdomyolysis associated with Legionella infection. Most cases were from non-English publications, which could not be adequately translated, limiting data extraction. A CK level increase of > 50 000 U/L was identified in only seven of the 23 patients (Box).3–9 A marked predominance of male sex was observed. All patients underwent dialysis except one, who was managed with intravenous mannitol and forced alkaline diuresis,3 but all survived.
In conclusion, while a moderate increase in CK concentration is common with Legionella infection, marked rhabdomyolysis with CK levels > 50 000 U/L leading to acute renal failure is rare. To our knowledge, this is the first case report of L. pneumophila from Australia with this association. Clinicians should be aware of this possible complication and maintain a high index of suspicion, since early recognition and prompt treatment will prove lifesaving.
Box –
Seven out of 23 reported cases had creatine kinase (CK) levels higher than 50 000 U/L3–9
|
Patient no. |
Year, journal |
Patient age in years |
Sex |
Location |
Creatinine at presentation (μmol/L) |
Maximum CK (U/L) |
Dialysis |
Outcome |
|||||||
|
|
|||||||||||||||
|
1 |
1983, Chest |
26 |
Male |
United States |
203 |
165 600 |
Yes |
Survived |
|||||||
|
2 |
1997, Enfermedades Infecciosas y Microbiología Clínica |
61 |
Male |
France |
91 |
202 900 |
No |
Survived |
|||||||
|
3 |
2002, Southern Medical Journal |
56 |
Male |
United States |
247 |
115 880 |
Yes |
Survived |
|||||||
|
4 |
2007, New York Medical Journal |
62 |
Male |
United States |
318 |
176 526 |
Yes |
Survived |
|||||||
|
5 |
2008, Journal of Clinical Pathology |
54 |
Male |
Japan |
450 |
52 000 |
Yes |
Survived |
|||||||
|
6 |
2011, Journal of Cardiology Cases |
58 |
Male |
Japan |
300 |
93 320 |
Yes |
Survived |
|||||||
|
7 |
2012, Chest |
42 |
Male |
United States |
167 |
110 355 |
Yes |
Survived |
|||||||
|
|
|||||||||||||||
Surviving accidental paraquat ingestion: a limited evidence zone
A 17-year-old youth accidentally ingested paraquat, a herbicide which is an uncommon but potentially highly lethal cause of poisoning. He was initially managed at a regional hospital where, upon advice from a Victorian Poisons Information Centre toxicologist, he was commenced on dexamethasone, continuous venovenous haemodiafiltration (CVVHDF), and N-acetyl cysteine (NAC) and sodium salicylate infusions. He was transferred to a transplant centre for consideration of lung transplantation where he was maintained on CVVHDF, dexamethasone and NAC for 2 weeks. Fortunately, despite ingestion of a potentially lethal dose of paraquat at 36.48 mg/kg, he recovered with mild restrictive respiratory deficits, steroid side effects, and oropharyngeal burns as sequelae.
Paraquat toxicity primarily damages the respiratory and renal systems. In the alveolar epithelium, absorbed paraquat concentrations can be up to 10–20 times the serum paraquat levels.1 Here it undergoes oxidation to form superoxide radicals leading to progressive pulmonary fibrosis. In the kidneys, paraquat induces acute tubular necrosis, which impairs its elimination and further increases lung concentrations. The ingestion of greater than 20 mg/kg of paraquat, which represents only 10–20 mL of a 20% w/w solution typically leads to acute kidney injury (AKI) and progressive pulmonary fibrosis causing death in 1–3 weeks.2
The diagnosis of paraquat poisoning may be made urgently by the semiquantitative urinary dithionite spot test. Knowing the serum paraquat concentration is useful for prognostication purposes, and five nomograms predicting outcome from 4 to 200 hours postingestion are available for use.3 However, none of these as yet alter clinical management.
The optimal management of paraquat poisoning, as with most pesticide poisonings, remains unclear. The patient in this report developed rapid AKI with creatinine levels increasing from a baseline of 90 μmol/L to 239 μmol/L within 28 hours of ingestion. For this, he was maintained on CVVHDF for 2 weeks, although the benefit of this treatment is unclear. Haemoperfusion, as opposed to continuous haemofiltration, is more efficacious at removing paraquat from the serum, and despite widespread clinical use the evidence for its use is mostly limited to animal studies.4 Paraquat induces an intense inflammatory reaction, so other treatment modalities have focused on immunosuppressive and antioxidant therapies. Small, underpowered studies have previously used pulsed methylprednisolone and cyclophosphamide followed by 2 weeks of dexamethasone, although cyclophosphamide has been shown to have little efficacy in small randomised controlled trials.5 Sodium salicylate and NAC both act in an anti-inflammatory manner, although the data are again mostly limited to animal studies.3
Paraquat toxicity remains an uncommon event in Australia. When paraquat poisoning is suspected, the involvement of a specialist toxicology service accessible through avenues such as the Poisons Information Australia provides an indispensable avenue for expertise to manage this limited evidence zone.
Toilet bowl palsy from prolonged prayer posture
An 18-year-old man was admitted to a Cambodian hospital with severe bilateral lower leg weakness and an acute kidney injury requiring renal replacement therapy. Three days before his hospitalisation, he had consumed tramadol hydrochloride and codeine phosphate, and injected heroin. While intoxicated in his hostel, he adopted a prayer posture (Box) and subsequently lost consciousness, remaining in this position for 8 hours on a tiled floor. Upon regaining consciousness, he was unable to stand due to a profound weakness in both legs that persisted for 3 days. During this time his urine changed to a cola colour although it was of normal volume. On the fourth day of ongoing symptoms, the patient sought medical care and was found to be in acute renal failure. He received two sessions of haemodialysis.
After his discharge from the intensive care unit, the patient’s mother escorted him to an Australian hospital. On presentation to the emergency department, he was oriented to time and place, but had persisting weakness, loss of sensation in his lower limbs and an ulnar nerve paresis. An isolated patch of paraesthesia over the forehead was noted at the site of contact with the ground. He had no features of uraemia. He was anuric and tests showed a creatinine level of 1040 μmol/L (reference interval [RI], 73–108 μmol/L) and a markedly elevated creatine kinase level of 123 000 U/L (RI, 46–171 U/L). Urine was positive for urine myoglobin of 2850 μg/L (RI, < 10 μg/L). A diagnosis of acute renal failure secondary to rhabdomyolysis was made.
The patient received haemodialysis for 5 hours on alternate days until Day 17, when his renal function had sufficiently improved. Despite daily physiotherapy the patient made minimal gains in his lower limb power, with ankle power of 0/5 bilaterally. Neurologists diagnosed the patient with bilateral sciatic nerve compression due to “toilet bowl palsy”. This was thought to be secondary to direct compression of the nerve, a posterior thigh compartment syndrome and a stretch radiculopathy. He was transferred to a rehabilitation unit for further care. Upon review at 2 months, he had improvement of proximal muscle power but bilateral foot drop persisted.
Toilet bowl palsy is a rare condition characterised by bilateral sciatic nerve palsy from maintaining a prolonged abnormal posture. It arises from a period of immobility while sitting on a hard surface and is often associated with substance abuse. It can be iatrogenic (positioning in surgery) or can occur spontaneously, usually from toilet seat entrapment,1–3 yoga positioning,1 overstretching1 or, as in this case, maintaining a prayer posture for a prolonged period of time. This case highlights the severe and often irreversible nerve damage arising from toilet bowl palsy.
Beware of blotting paper hallucinogens: severe toxicity with NBOMes
Clinical record
16-year-old male presented to the emergency department after ingesting what he believed to be LSD (lysergic acid diethylamide) on red blotting paper while camping with friends in rural New South Wales in late 2014. He had no past medical or mental health history, and was taking no regular medications. He had three seizures before arriving in the ED, where his Glasgow coma scale score was 9. He had a fourth seizure about 1 hour after presenting, and was given 5 mg midazolam intravenously. His initial venous blood gas parameters were: pH 6.93 (reference range [RR], 7.35–7.45); PCO2, 120 mmHg (RR, 35–48 mmHg); and base excess, −7 (RR, 0.5–1.6). He was then intubated, ventilated, paralysed with rocuronium, and sedated with morphine/midazolam for transfer to a tertiary intensive care unit. His heart rate was 70 bpm, his blood pressure 130/60 mmHg, and he was afebrile after intubation. Over the next 3 hours and before medical retrieval, his blood gases normalised with improved ventilation (pH 7.4; PCO2, 29.6 mmHg).
He had no further seizures after his transfer to the tertiary intensive care unit. His overnight urine output was initially reduced; this improved with increased fluid replacement. On arrival at the intensive care unit, his blood parameters were: white cell count, 16.3 × 109/L (RR, 4–11 × 109/L); neutrophils 12.1 × 109/L (RR, 1.7–8.8 × 109/L); haemoglobin, 136 g/L (RR, 130–180 g/L); platelets, 198 × 109/L (RR, 150–400 × 109/L); sodium, 142 mM (RR, 134–145 mM); potassium, 3.9 mM (RR, 3.5–5.0 mM); and creatinine, 108 mM (64–104 mM). He remained haemodynamically stable and was extubated the following day. He was transferred to the paediatric ward, and on Day 2 his creatinine and creatine kinase levels were rising, with normal urine output (Figure). Except for some initial nausea that lasted for 24 hours after extubation, he had no other symptoms over the next 3 days, and experienced no hallucinations or agitation. His creatinine levels peaked at 246 mM [RR, 64–104 mM] 37.5 hours after ingestion, and his creatine kinase levels peaked at 34 778 U/L (RR, 1–370 U/L) 90 hours after ingestion. He was discharged well on Day 5 without complications.
NBOMe assays are not currently part of routine emergency toxicology testing; worldwide, only a few forensic and commercial laboratories offer qualitative NBOMe testing in blood or urine. Blood specimens from the patient were sent to the Department of Pathology at Virginia Commonwealth University (USA) for NBOMe detection and quantification. The specimens were tested by previously validated high-performance liquid chromatography/mass spectrometry assays.1,2 25B-NBOMe was detected in the blood specimen at a concentration of 0.089 μg/L, 22 hours after ingestion.
Dimethoxyphenyl-N-[(2-methoxyphenyl)methyl]ethanamine derivatives (NBOMes) are a novel class of potent synthetic hallucinogens originally developed as 5-HT2 receptor agonists for research purposes, but which have become available as recreational drugs in the past few years.3 They are available under a number of street names, including “N-bombs”, and are often sold as “acid” or “LSD” on blotting paper, as a powder, or as blue tablets (“blue batman”). They have been increasingly associated over the past 2 years with deaths and severe toxicity in North America and Europe.3–5 Most reports have concerned 25I-NBOMe intoxication, and there is much less information on the 25B- and 25C-NBOMe derivatives.2,6,7 While difficult to assess because of the sparse number of reports, 25B-NBOMe may be more toxic than the more commonly reported 25I-NBOMe.3,4 Our case is consistent with previous reports of severe NBOMe toxicity, with agitation, tachycardia and mild hypertension, seizures, rhabdomyolysis and acute kidney injury.3
There have been few reports of NBOMe poisoning in Australia, and only one report of a fatality.8 Most reports in Australia have been in the popular media, describing the presence of NBOMes in this country. There is limited information available to health care professionals about their potential toxicity. An international online survey in 2012 found that NBOMes were being used in Australia, although not as commonly as in the United States.5 NBOMes are reported to be relatively inexpensive, and are usually purchased over the internet. For this reason, as in our case, intoxicated NBOMe users may present to rural and smaller regional hospitals. As in other reports, our patient believed he had taken “acid” or LSD. The one reported death in Western Australia involved a woman who had inhaled a white powder she thought to be “synthetic LSD”; she began behaving oddly, before collapsing and dying.8 In comparison with the dramatic systemic effects seen in our case and those described in the literature, LSD is not associated with such severe medical complications.9
NBOMe toxicity is characterised by hallucinations and acute behavioural disturbance, with seizures, rhabdomyolysis and acute kidney injury in more severe cases.3,4,6,9,10 Our patient was postictal when he presented, and required immediate sedation and intubation, after which he was reported to have a normal heart rate and blood pressure. Rising creatinine and creatine kinase levels were recognised on the medical ward after the patient had been extubated.
Previous reviews3,4 suggested that there are two different presentation types of NBOMe toxicity: one form dominated by hallucinations and agitation, and another involving more severe medical complications. Patients presenting with the first type should be managed in a similar manner to other patients with acute behavioural disturbance, including verbal de-escalation and oral or parenteral sedation as required.11 In many cases, these patients will present with undifferentiated behavioural disturbance, and only the persistence of hallucinations or agitation and the history given by the patient will suggest the diagnosis. In patients with more severe medical complications, directed supportive care is appropriate, including intubation and ventilation for coma, and fluid replacement for rhabdomyolysis and acute renal impairment. Serial electrolyte, creatinine and creatine kinase measurements should be made in all cases to identify these complications and to monitor the progress of the patient. Further, such investigations may potentially play a role in identifying NBOMe as a cause in patients who present with undifferentiated agitation and hallucinations lasting 24 hours or more.
Lessons from practice
-
Dimethoxyphenyl-N-[(2-methoxyphenyl)methyl]ethanamine derivatives (NBOMes) are hallucinogenic substances that have become available as drugs of misuse in the past few years.
-
NBOMe toxicity can cause acute behavioural disturbance, and in severe cases can cause seizures, rhabdomyolysis and acute kidney injury.
-
NBOMes may be distributed as lysergic acid diethylamide (LSD) or “acid” on blotting paper.
-
Treatment is supportive, including sedation for agitation and intravenous fluid therapy for rhabdomyolysis and acute renal failure.
Clinicians need to be aware that newer synthetic hallucinogens, such as NBOMes, are available in Australia, and that patients may believe them to be “acid” or LSD. NBOMes cause prolonged agitation and hallucinations and, in more severe cases, seizures, rhabdomyolysis and acute kidney injury.

 more_vert
more_vert