The known The incidence of young-onset colorectal cancer (yCRC) is increasing in developed countries.
The new The incidence of yCRC is currently stable in NSW. Rectal and distal colon cancer is more common in younger patients. Cancer-specific survival is better for younger than for older patients, despite more advanced disease at diagnosis.
The implications 6% of all CRC occurs in people under 50 years of age, who are not eligible for the National Bowel Cancer Screening Program. Health professionals should be alert to alarm symptoms in younger patients that would allow earlier detection and treatment of CRC.
In 2012, more than 1.3 million people had colorectal cancer (CRC) worldwide, and there were 694 000 associated deaths.1 While the incidence of CRC is increasing rapidly in countries with transitioning economies, probably because of increased exposure to risk factors associated with rising prosperity, including dietary and lifestyle factors,2 it appears to have plateaued in developed countries.3,4 However, recent studies in the United States and Europe5–7 have identified an alarming increase in incidence among adults under 50 years of age; indeed, 2–9% of all new cases of CRC worldwide are diagnosed in people under 50.4,8 In the US, certain ethnic groups, including African Americans and Hispanics, are disproportionately represented in this age group.9,10
Australia, which shares a similar Western lifestyle with the US and Europe, has the highest incidence of CRC in the world.1 However, population-based data on incidence trends and outcomes for patients under 50 years of age in Australia are limited. Conflicting findings have been reported from studies that have evaluated different time periods, and that were limited by their use of aggregated data11 or the lack of survival outcomes.12 Therefore, our aim was to conduct a population-based study that assessed the incidence of CRC in younger adults in New South Wales, the demographic and clinico-pathological characteristics of these patients, and their survival.
Methods
Study population
The study population included all cases of adenocarcinoma of the colon and rectum diagnosed in NSW (resident population [2008], 7 million) between 1 January 2001 and 31 December 2008.
Data sources
Data on newly diagnosed cases of CRC were obtained from the NSW Central Cancer Registry, a statutory registry that contains information on patient demographic characteristics, cancer diagnosis, and date and cause of death for each new case of cancer diagnosed in NSW, based on mandatory notifications by public and private hospitals. At the time of extraction in 2012, complete data were available only for 2001–2008. Mortality data were obtained from death registrations by the NSW Registry of Births Deaths and Marriages to 2012, allowing calculation of 5-year survival for the entire cohort. The diagnosis, topography and morphology for each cancer diagnosis were coded according to the International Classification of Diseases, version 10, Australian modification (ICD-10-AM) and the third edition of the World Health Organization International Classification of Diseases for Oncology (ICD-O-3).
Study outcomes and factors
The primary outcomes of interest were the incidence of CRC, trends in incidence, cancer spread at presentation, and cancer-specific survival. The main study factor of interest was age at diagnosis, dichotomised into patients under 50 years of age (young-onset colorectal cancer, yCRC) and patients aged 50 years or more. Explanatory variables examined included CRC, stratified into cancers of the colon (ICD-10-AM C18.0, C18.2–C18.9; with right colon defined as caecum [C18.0], ascending colon [C18.2] and hepatic flexure [C18.3]), the rectosigmoid (ICD-10-AM C19.9), and the rectum (ICD-10-AM C20.9); age at diagnosis; sex; country of birth; socio-economic status; and geographic remoteness. Socio-economic status, based on the postcode of residence, was derived from the Australian Bureau of Statistics’ Index of Relative Socio-economic Disadvantage13 and aggregated into quintiles, ranging from “least disadvantaged” (quintile 1) to the “most disadvantaged” (quintile 5). Similarly, area of residence was assigned to one of five categories (major cities, inner regional, outer regional, remote and very remote), based on the Accessibility and Remoteness Index of Australia (ARIA+).14 Disease spread, defined as the maximum extent of disease at diagnosis, was assessed; spread at diagnosis was defined as the maximum extent of disease, and is reported as localised, regional, distant (metastatic), or unknown.
Statistical analyses
Overall age-specific CRC incidence rates were calculated, stratified by age group and CRC type. Rates were then standardised to the NSW population at 30 June 2001 and expressed as numbers per 100 000 people. Poisson regression assessed the average annual linear trend in incidence rates of CRC by diagnosis age group, corrected for overdispersion. Crude associations between diagnosis age group and each potential risk factor for cancer were assessed in cross-tabulations and χ2 tests. Unadjusted median and 5-year survival estimates for cancer-specific mortality were determined, and presented as product limit Kaplan–Meier survival curves. The association between age at time of diagnosis and cancer-specific mortality was assessed by multivariable Cox regression, adjusted for potential confounding by explanatory variables. Proportional hazards assumptions were assessed graphically and statistically, including testing time-dependent covariates, with final models satisfying the assumptions. Adjusted hazard ratios (aHRs) and 95% confidence intervals [CIs] were calculated. Analyses were conducted in SAS 9.4 (SAS Institute) and Stata/IC 13.0 (StataCorp). P < 0.05 was deemed statistically significant.
Ethics approval
This study was approved by the NSW Population and Health Services Research Ethics Committee (reference, 2012-06-020).
Results
Demographic characteristics of the study population
A total of 32 178 new cases of colorectal adenocarcinoma were diagnosed in NSW during 2001–2008. At diagnosis, 2001 patients (6.2%) were under 50 years of age (including 1491 [74.5%] aged 40–49 years); 30 177 patients (93.8%) were 50 years old or more. Most patients in each age group were men, and their proportion was greater among the older patients (≥ 50 years, 55.7%; < 50 years, 51.5%). A greater proportion of patients with yCRC lived in the city (72.1% v 66.9% for older patients), were born outside Australia (31.1% v 28.9%), and lived in postcodes in the lowest socio-economic quintile (19.5% v 18.1%; Box 1).
Trend in incidence of CRC
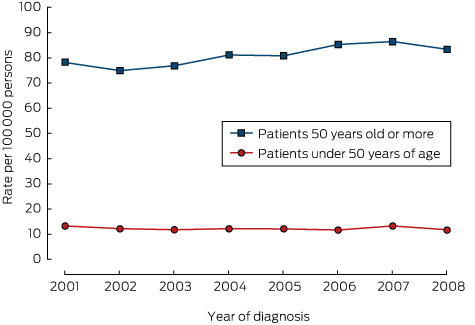
There was no significant change in the overall incidence of yCRC (2001, 13.7 cases per 100 000 population; 2008, 11.8 per 100 000; P = 0.26; Box 2). Further, there was no change in incidence in younger subsets of the yCRC cohort (under 40 years of age, P = 0.17; 40–49 years of age, P = 0.59; data not shown). For people over 50 years of age, there was a small increase in the incidence of CRC, from 79.0 per 100 000 population in 2001 to 83.4 per 100 000 in 2008 (P = 0.045; Box 2). There were no significant changes in the incidence of CRC in specific locations over time (colon, P = 0.21; rectosigmoid, P = 0.44; rectum, P = 0.85; data not shown).
Tumour site, spread and histopathology
In patients with yCRC, most tumours were in the distal colon and rectum; 71.5% of tumours were located distal to the splenic flexure. Rectal cancer was more common in those with yCRC than in older patients (34.4% v 26.0%). In contrast, tumours in the right colon were more frequent in patients over 50 years of age (28.4% v 19.3%). A higher proportion of patients with yCRC had advanced disease; distant disease was more common than in patients over 50 years of age (21.2% v 15.3%), and localised disease more frequent in patients older than 50 years (35.3% v 28.6%; Box 1).
Survival
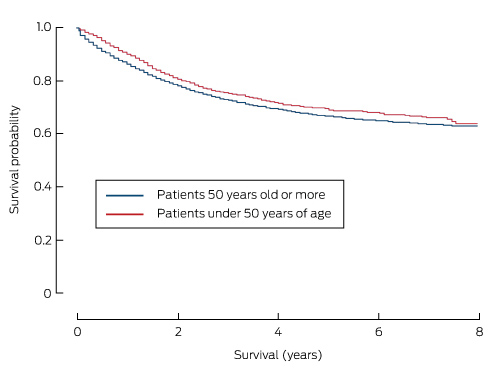
Death attributed to CRC was the most common cause of death in both age groups (Box 1). Overall 5-year survival was significantly higher for patients with yCRC (67.1% [95% CI, 64.5–69.6%] v 55.8% [95% CI, 55.0–56.4%] for those over 50 years of age; P < 0.001). As a result, patients with yCRC had a 33% lower risk of dying from their disease than those over 50 years of age (adjusted hazard ratio [aHR], 0.67; 95% CI, 0.61–0.74). Additionally, 5-year cancer-specific survival was also slightly higher for patients with yCRC (68.8% [95% CI, 66.2–71.2%]) than for those over 50 years of age (66.3% [95% CI, 65.6–67.0%]; P < 0.001; Box 3), but was similar to 5-year cancer-specific survival for patients aged 50–59 years (69.7%; 95% CI, 68.0–71.3%).
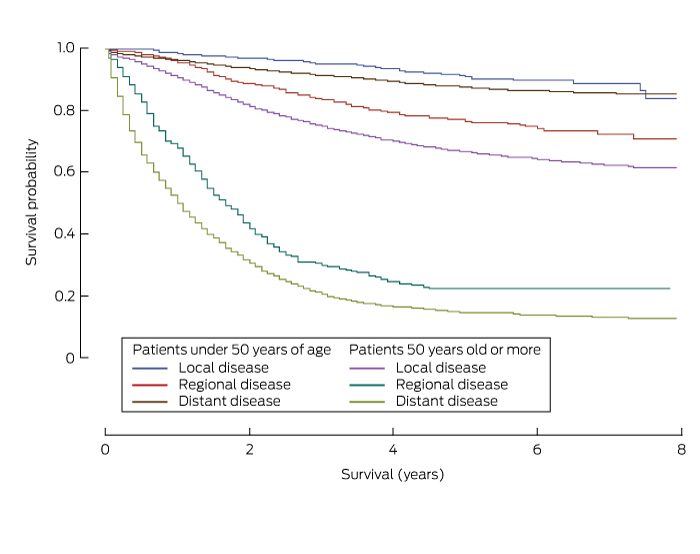
Five-year survival was consistently higher for patients with yCRC than for patients aged 50 years or more for each of the clinical, demographic and pathological parameters analysed (Box 4). Survival was also greater for patients with colon cancer than for those with rectosigmoid cancer, survival with which was in turn higher than that for patients with rectal cancer (Box 4). Additionally, 5-year survival varied significantly with spread of disease; it was highest for those with localised cancer (yCRC, 90.9% [95% CI, 87.2–93.5%]; over 50 years of age, 87.6% [95% CI, 86.7–88.4%]) than for those with regional disease (yCRC, 76.6% [95% CI, 72.7–80.0%]; over 50 years of age, 66.7% [95% CI, 65.6–67.8%]) or distant disease (yCRC, 22.3% [95% CI, 17.5–27.5%]; over 50 years of age, 14.6% [95% CI, 13.3–16.0%]) (Box 4, Box 5).
Factors associated with cancer-specific mortality in patients with yCRC
In patients with yCRC, cancer-specific mortality was higher for those from disadvantaged areas (quintile 1 v quintiles 2–4: aHR, 1.39; 95% CI, 1.16–1.92), those with increased spread of disease (regional spread v localised: aHR, 2.97; 95% CI, 2.07–4.24; distant spread v localised: aHR, 17.60; 95% CI, 12.49–24.81), and those who did not undergo surgery within 3 months of diagnosis (aHR, 1.88; 95% CI, 1.53–2.29) (Box 6). In contrast, cancer-specific mortality was lower for patients with yCRC who were born outside Australia (aHR, 0.80; 95% CI, 0.66–0.98) (Box 6).
Discussion
This population-based study shows that there was no increase during 2001–2008 in the incidence of CRC in patients less than 50 years of age. Young patients with CRC were more often from disadvantaged and urban backgrounds and born overseas than older patients with CRC, and they presented more frequently with left-sided and rectal cancers, with greater spread at diagnosis than those aged 50 years or more. However, adjusted 5-year survival for patients with yCRC was better than for older patients, and the risk of cancer-specific mortality was also significantly lower.
The overall incidence of CRC has plateaued in many developed countries.4 Indeed, both incidence and mortality have fallen in the US, and this has been attributed to screening programs, reducing exposure to risk factors, and better treatment.15 Despite these improvements, the incidence of yCRC is rising in the US5 and Europe,6 perhaps the consequence of young people being increasingly exposed earlier in life to known risk factors, including obesity, inactivity, fast food consumption, and diabetes.4,7
Australia has the highest incidence of CRC in the world1 and its urban lifestyle is similar to that of the US and Europe. Nevertheless, we did not find an increase in the incidence of yCRC across the study period. Our finding is consistent with that of another, smaller population-based study in Victoria, which found that 7% of all cases of colorectal cancer diagnosed during 2000–2010 were in people under 50 years of age, and that there had been no increase in incidence.12 In contrast, a recent analysis of aggregated 5-year data from both the National and the South Australia Cancer Registries found an increase in incidence in subgroups of patients with yCRC; specifically, an increase was noted for patients under 40 years of age, although the incidence was stable or falling in those over 40 years of age.11 This difference in reported trend is probably explained by differences in the baseline incidence rate during the two relevant study periods (1990–2010 in the South Australian study, 2001–2008 in our study).
The apparent lack of an increase in the incidence of yCRC in NSW cannot be explained by information available to our study. However, the impact of rising rates of obesity and diabetes in Australian children16 on the incidence of CRC in young adults may yet to be realised. Further, certain ethnic groups are disproportionately represented among American patients with yCRC,9,10 but differences in immigration patterns mean that the ethnic make-up of the Australian population is different.
In our study, a higher proportion of patients with yCRC than of older patients lived in cities and in lower socio-economic status postcodes. Low socio-economic status and urban residence, in addition to migration to high risk countries, have all been identified as risk factors for CRC.3,17 Patients with yCRC were more likely to present with greater spread of disease at diagnosis, with 22% having distant disease, consistent with studies that also found that younger patients had often had symptoms for several months.18,19 We were unable to determine the prevalence of symptoms in patients with yCRC, but more than 70% of tumours were distal to the splenic flexure and 34% were in the rectum, consistent with findings of previous studies,7 including one in Australia.10 Large population studies have identified that survival is poorer for patients with rectal cancers than for those with right colon tumours.20
The screening of asymptomatic individuals over 50 years of age has been credited with reducing the incidence and mortality of CRC,15 and it has been argued that screening programs should be expanded to include younger adults.21 However, we found that the rates of CRC in Australia are much lower in adults under 50 years of age (12 per 100 000 population in 2008) than in those over 50 years of age (83 per 100 000). The cost of screening for CRC in Australia is estimated to be $25 000–$41 667 for each year of life gained.22 However, the cost of each life year gained would be higher were screening extended to 40–49-year-old people, as there is a lower incidence of adenoma in younger patients.23
yCRC has been described as having a particularly aggressive phenotype, with adverse pathological features and lower survival.18,24 We found, however, that stage-adjusted cancer-specific survival in patients with yCRC was better than for older patients. Indeed, the risk of CRC-related death was 33% lower, and 5-year survival was greater. Increased survival may be the result of otherwise better general health than in older patients, or of the greater likelihood of being selected for and completing adjuvant chemotherapy treatment.17 Although encouraging, longer term (10-year) survival data are needed to confirm that this benefit is sustained, particularly as many patients with yCRC have advanced disease at diagnosis, and their condition may subsequently deteriorate if disease recurs after more than 5 years.25
The strength of our investigation was that it was a large population-based study of almost 7 million people over nearly a decade, employing robust data collection methods and long term survival data. However, there were some limitations. We did not have detailed clinical or pathology reports for each patient, and we were not able to determine the contribution of genetic conditions to CRC in the younger patients. Although major genetic components are implicated in fewer than 5% of all CRC cases, this proportion may be higher for patients with yCRC.
In conclusion, the incidence of yCRC in NSW did not increase during 2001–2008. Patients with yCRC accounted for 6% of all CRC cases; they presented with distal tumours and advanced disease more often than did older patients with CRC. People under 50 years of age are not eligible for the National Bowel Cancer Screening Program, so health professionals should be alert to alarm symptoms in younger patients (eg, persistent unexplained bleeding per rectum or change in bowel habit) that would allow earlier detection and treatment of CRC.
Box 1 –
Demographic characteristics of the study population, and clinico-pathologic characteristics of newly diagnosed cases of colorectal cancer, New South Wales, 2001–2008, by age group
|
Demographic and clinical characteristics
|
Less than 50 years old
|
50 years old or more
|
P
|
Total
|
|
Number
|
%
|
Number
|
%
|
Number
|
%
|
|
|
Total number of patients
|
2001
|
30 177
|
|
32 178
|
|
Age group
|
|
|
|
|
|
|
|
|
< 30 years
|
109
|
5.5%
|
—
|
—
|
|
109
|
0.3%
|
|
30–39 years
|
401
|
20.0%
|
—
|
—
|
|
501
|
1.2%
|
|
40–49 years
|
1491
|
74.5%
|
—
|
—
|
|
1491
|
4.6%
|
|
50–59 years
|
—
|
—
|
4747
|
15.7%
|
|
4747
|
14.8%
|
|
60–69 years
|
—
|
—
|
8619
|
28.6%
|
|
8619
|
26.8%
|
|
70–79 years
|
—
|
—
|
10 127
|
33.6%
|
|
10 127
|
31.5%
|
|
80–89 years
|
—
|
—
|
6003
|
19.9%
|
|
6003
|
18.7%
|
|
≥ 90 years
|
—
|
—
|
681
|
2.3%
|
|
681
|
2.1%
|
|
Sex
|
|
|
|
|
0.002
|
|
|
|
Male
|
1030
|
51.5%
|
16,810
|
55.7%
|
|
17 840
|
55.4%
|
|
Female
|
971
|
48.5%
|
13,367
|
44.3%
|
|
14 338
|
44.6%
|
|
Country of birth
|
|
|
|
|
0.008
|
|
|
|
Australia
|
1378
|
68.9%
|
21 438
|
71.0%
|
|
22 816
|
70.9%
|
|
Outside Australia
|
623
|
31.1%
|
8739
|
28.9%
|
|
9362
|
29.1%
|
|
Geographic remoteness
|
|
|
|
|
< 0.001
|
|
|
|
Major cities
|
1443
|
72.1%
|
20 202
|
66.9%
|
|
21 645
|
67.3%
|
|
Inner regional
|
414
|
20.7%
|
7442
|
24.7%
|
|
7856
|
24.4%
|
|
Outer regional
|
131
|
6.6%
|
2381
|
7.9%
|
|
2512
|
7.8%
|
|
Remote/very remote
|
13
|
0.6%
|
152
|
0.5%
|
|
165
|
0.5%
|
|
Socio-economic status (quintiles)
|
|
|
|
|
< 0.001
|
|
|
|
1 (least disadvantaged)
|
392
|
19.6%
|
6118
|
20.3%
|
|
6510
|
20.2%
|
|
2
|
423
|
21.1%
|
5206
|
17.3%
|
|
5629
|
17.5%
|
|
3
|
366
|
18.3%
|
6334
|
21.0%
|
|
6700
|
20.8%
|
|
4
|
430
|
21.5%
|
7064
|
23.4%
|
|
7494
|
23.3%
|
|
5 (most disadvantaged)
|
390
|
19.5%
|
5455
|
18.1%
|
|
5845
|
18.2%
|
|
Spread at diagnosis
|
|
|
|
|
< 0.001
|
|
|
|
Localised
|
572
|
28.6%
|
10 651
|
35.3%
|
|
11 223
|
34.9%
|
|
Regional
|
861
|
43.0%
|
12 605
|
41.8%
|
|
13 466
|
41.8%
|
|
Distant
|
444
|
21.2%
|
4621
|
15.3%
|
|
5065
|
15.7%
|
|
Unknown
|
124
|
6.2%
|
2300
|
7.6%
|
|
2424
|
7.5%
|
|
Tumour location
|
|
|
|
|
< 0.001
|
|
|
|
Colon
|
|
|
|
|
|
|
|
|
Right colon
|
386
|
19.3%
|
9029
|
28.4%
|
|
8967
|
27.9%
|
|
Transverse
|
104
|
5.2%
|
2284
|
7.1%
|
|
2260
|
7.0%
|
|
Left colon
|
120
|
6.0%
|
1885
|
5.9%
|
|
1898
|
5.9%
|
|
Sigmoid
|
420
|
21.0%
|
6415
|
19.9%
|
|
6426
|
20.0%
|
|
Unspecified
|
80
|
4.0%
|
1425
|
4.5%
|
|
1425
|
4.4%
|
|
Rectosigmoid
|
203
|
10.1%
|
2476
|
8.2%
|
|
2679
|
8.3%
|
|
Rectal
|
688
|
34.4%
|
7835
|
26.0%
|
|
8523
|
26.5%
|
|
Cause of death
|
|
|
|
|
< 0.001
|
|
|
|
Missing/alive
|
1490
|
74.5%
|
19 541
|
64.8%
|
|
21 031
|
65.4%
|
|
Colon cancer
|
259
|
12.9%
|
5121
|
17.0%
|
|
5380
|
16.7%
|
|
Rectal cancer
|
225
|
11.2%
|
2555
|
8.5%
|
|
2780
|
8.6%
|
|
Other cancer
|
7
|
0.4%
|
647
|
2.3%
|
|
654
|
2.0%
|
|
Non-cancer death
|
13
|
0.7%
|
2194
|
7.7%
|
|
2207
|
6.9%
|
|
Unknown cause of death
|
7
|
0.3%
|
119
|
0.4%
|
|
126
|
0.4%
|
|
|
|
Box 2 –
Incidence of colorectal cancer, New South Wales, 2001–2008, by age group
Box 3 –
Cancer-specific survival curves for patients with colorectal cancer, New South Wales, 2001–2008, by age group
Box 4 –
Five-year survival rates for patients with colorectal cancer, New South Wales, 2001–2008
|
Demographic and clinical characteristics
|
Five-year survival (95% CI)
|
P
|
|
Less than 50 years old
|
50 years old or more
|
|
|
Age group
|
|
|
< 0.001
|
|
< 30 years
|
65.6% (52.6–75.8%)
|
|
|
|
30–39 years
|
70.1% (64.3–75.1%)
|
|
|
|
40–49 years
|
68.6% (65.5–71.4%)
|
|
|
|
50–59 years
|
|
69.7% (68.0–71.3%)
|
|
|
60–69 years
|
|
70.2% (69.0–71.5%)
|
|
|
70–79 years
|
|
66.6% (65.4–67.8%)
|
|
|
80–89 years
|
|
58.8% (57.1–60.5%)
|
|
|
≥ 90 years
|
|
45.1% (38.9–51.1%)
|
|
|
Sex
|
|
|
0.03
|
|
Male
|
67.7% (63.9–71.1%)
|
65.8% (64.9–66.8%)
|
|
|
Female
|
69.2% (65.6–72.7%)
|
66.9% (65.9–67.9%)
|
|
|
Country of birth
|
|
|
< 0.001
|
|
Australia
|
66.9% (63.7–69.9%)
|
65.6% (64.8–66.4%)
|
|
|
Outside Australia
|
72.9% (68.4–76.9%)
|
68.1% (66.8–69.3%)
|
|
|
Geographic remoteness
|
|
|
< 0.001
|
|
Urban
|
69.6% (66.6–72.4%)
|
66.9% (66.0–67.7%)
|
|
|
Rural
|
66.1% (61.1–70.7%)
|
65.3% (64.0–66.4%)
|
|
|
Socio-economic status
|
|
|
< 0.001
|
|
Quintile 1 (least disadvantaged)
|
73.4% (67.6–78.3%)
|
68.8% (67.3–70.2%)
|
|
|
Quintiles 2–5
|
67.6% (64.7–70.3%)
|
65.7% (64.9–66.4%)
|
|
|
Cancer type
|
|
|
0.007
|
|
Colon
|
69.9% (66.4–73.0%)
|
66.5% (65.6–67.3%)
|
|
|
Rectosigmoid
|
67.7% (58.5–75.4%)
|
66.9% (64.5–69.3%)
|
|
|
Rectal
|
66.0% (61.2–70.3%)
|
65.7% (64.3–67.1%)
|
|
|
Spread at diagnosis
|
|
|
< 0.001
|
|
Localised
|
90.9% (87.2–93.5%)
|
87.6% (86.7–88.4%)
|
|
|
Regional
|
76.6% (72.7–80.0%)
|
66.7% (65.6–67.8%)
|
|
|
Distant
|
22.3% (17.5–27.5%)
|
14.6% (13.3–16.0%)
|
|
|
Colorectal surgery within 3 months of diagnosis
|
|
|
< 0.001
|
|
Yes
|
73.0% (70.2–75.6%)
|
70.0% (69.2–70.7%)
|
|
|
No
|
53.4% (47.4–59.1%)
|
52.8% (51.3–54.3%)
|
|
|
|
|
Box 5 –
Cancer-specific survival curves for patients with colorectal cancer, New South Wales, 2001–2008, by stage of diagnosis and age group
Box 6 –
Factors associated with cancer-specific mortality for patients with young-onset colorectal cancer, New South Wales, 2001–2008
|
Demographic and clinical characteristics
|
Deaths
|
Hazard ratio (95% CI)
|
|
Number
|
%
|
Crude
|
Adjusted*
|
|
|
Number of patients
|
481
|
|
|
|
|
Age group (years)
|
|
|
|
|
|
< 30 years
|
27
|
5.6%
|
1.11 (0.75–1.65)
|
1.26 (0.85–1.86)
|
|
30–39 years
|
98
|
20.3%
|
0.99 (0.79–1.24)
|
0.94 (0.75–1.17)
|
|
40–49 years
|
356
|
74.0%
|
1
|
1
|
|
Sex
|
|
|
|
|
|
Male
|
250
|
52.0%
|
1.02 (0.86–1.22)
|
1.07 (0.90–1.29)
|
|
Female
|
231
|
48.0%
|
1
|
1
|
|
Country of birth
|
|
|
|
|
|
Australia
|
349
|
72.6%
|
1
|
1
|
|
Outside Australia
|
132
|
27.4%
|
0.81 (0.66–0.99)†
|
0.80 (0.66–0.98)†
|
|
Geographic remoteness
|
|
|
|
|
|
Urban
|
337
|
70.1%
|
1
|
1
|
|
Rural
|
144
|
29.9%
|
1.14 (0.93–1.38)
|
0.95 (0.75–1.14)
|
|
Socio-economic status
|
|
|
|
|
|
Quintile 1 (least disadvantaged)
|
78
|
16.2%
|
1
|
1
|
|
Quintiles 2–5
|
403
|
83.8%
|
1.33 (1.05–1.70)†
|
1.39 (1.16–1.92)†
|
|
Cancer type
|
|
|
|
|
|
Colon
|
258
|
53.6%
|
1
|
1
|
|
Rectosigmoid
|
47
|
9.8%
|
0.97 (0.71–1.33)
|
0.82 (0.60–1.13)
|
|
Rectal
|
176
|
36.6%
|
1.09 (0.90–1.32)
|
1.18 (0.97–1.44)
|
|
Spread at diagnosis
|
|
|
|
|
|
Localised
|
38
|
7.9%
|
1
|
1
|
|
Regional
|
146
|
30.4%
|
2.83 (1.98–4.04)†
|
2.97 (2.07–4.24)†
|
|
Distant
|
271
|
56.3%
|
17.9 (12.7–25.2)†
|
17.6 (12.5–24.8)†
|
|
Colorectal surgery within 3 months of diagnosis
|
|
|
|
|
|
Yes
|
320
|
66.5%
|
1
|
1
|
|
No
|
161
|
33.4%
|
2.16 (1.78–2.61)†
|
1.88 (1.53–2.29)†
|
|
|
* Adjusted for all other factors listed in the first column. † P < 0.05.
|

 more_vert
more_vert