The known Post-traumatic stress disorder (PTSD) is associated with poor physical health, but few analyses of the relationship have been controlled for trauma exposure.
The new This is the first study to find that several comorbidities are associated with PTSD in older veterans by comparing the veterans with a trauma-exposed control group. These associations remained after adjusting for potential confounding factors.
The implications PTSD should be viewed as a systemic disorder rather than a purely psychological disorder. Integrated health care strategies for improving psychological and physical health, as well as controlling risk factors, could improve the quality of life and survival of patients.
Post-traumatic stress disorder (PTSD) is a disabling mental health condition that can develop following exposure to a traumatic event. People with PTSD experience a range of psychological symptoms, including intrusive memories, significant changes in cognition, mood, arousal and reactivity, as well as persistent avoidance of reminders of the traumatic event. The disorder can compromise personal behaviour, including the ability to maintain employment, relationships, and general self-care.
PTSD is common among combat veterans. Investigation of Australian veterans of the Vietnam War has found a lifetime prevalence of 20.9%,1 similar to the estimated prevalence of war-zone PTSD among United States Vietnam War veterans of 17.0%.2 The prevalence of PTSD among Australians who served in Iraq or Afghanistan is 16.5%,3 and the 12-month prevalence among veterans of Australian peacekeeping missions is as high as 16.8%.4
In addition to significant psychological effects, PTSD is associated with considerable physical comorbidity.5–7 Veterans with PTSD are more likely to suffer from physical illness and chronic disease than the general population or veterans without PTSD.5,7 Specifically, PTSD has been associated with gastrointestinal disease,7 respiratory disease,8 musculoskeletal, renal and autoimmune diseases,9 and with cardiovascular disease (CVD); veterans with PTSD have a higher prevalence of myocardial infarction (MI) and increased CVD mortality.10 PTSD is also associated with sleep disturbances, with nightmares and insomnia being common. More recently, an increased prevalence of obstructive sleep apnoea (OSA) was also reported.11 Responses to chronic stress in PTSD, such as increased substance misuse, poor diet and inactivity, have the potential to further harm health.12
Many studies investigating the impact of PTSD on health have examined the prevalence of certain disorders in people with PTSD. However, most have relied on self-reported data, and have not applied a clear definition of trauma exposure in their control groups, making it unclear whether the measured health effects were associated with PTSD or with trauma. We therefore compared self-reported and objective clinical health data for a group of Australian Vietnam War veterans with PTSD and a control group of veterans exposed to trauma but not suffering PTSD.
Methods
Participants
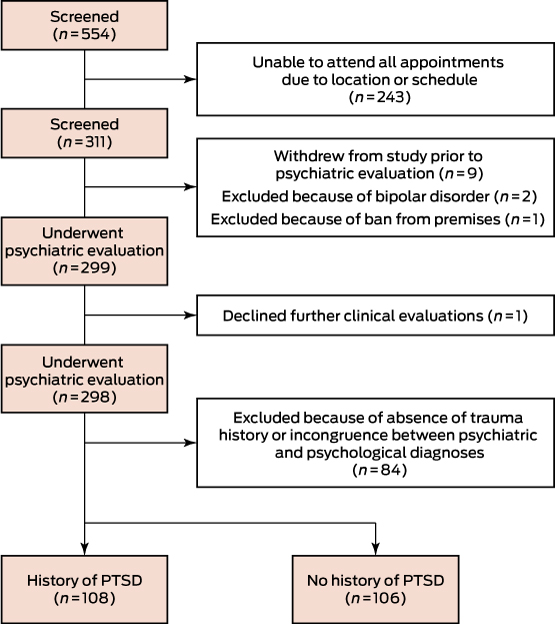
Male veterans who had served in the Australian or New Zealand armed services in Vietnam during the Vietnam War were recruited between February 2014 and July 2015 by a specialised veteran mental health unit at Greenslopes Private Hospital in Brisbane through the Gallipoli Medical Research Foundation and Returned and Services League of Australia (RSL) websites, through RSL publications and newspaper and television advertisements, and by word of mouth. Written informed consent was obtained from participants in advance. Enrolment was stratified by likely PTSD diagnosis. The study was conducted at the Gallipoli Medical Research Institute.
Data collection, management and quality assurance
Participants were assessed by a psychiatrist experienced in diagnosing and treating veterans with PTSD; they were classified as ever having had PTSD (currently or in the past) or not. Participants also underwent assessment by a psychologist using the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5), the Depression Anxiety Stress Scale (DASS-21), the Alcohol Use Disorders Identification Test (AUDIT), and the Montreal Cognitive Assessment (MoCA). A structured comprehensive medical history was obtained and a clinical examination conducted by a medical officer. Sleep information was obtained with structured questionnaires, the Berlin Questionnaire for assessing risk of OSA, and the Epworth Sleepiness Scale (ESS). A brief description of all validated questionnaires is provided in the online Appendix 1, table 1.
Electrocardiography (ECG), respiratory function tests, liver transient elastography, fasting abdominal ultrasonography, and computed tomography for coronary artery calcium (CAC) were conducted, and fasting blood pathology assessed.
All data were entered and stored in a Microsoft Excel database. Data quality was ensured by routine database checks and a random chart audit.
Statistical analysis
Outcomes statistically associated with PTSD status were identified, followed by regression analysis of outcomes associated with PTSD to exclude the effects of confounding variables and to evaluate the association of depression or anxiety symptoms with outcomes. Odds ratios (ORs) were calculated for binary data outcomes, and continuous data were compared in unpaired Student’s t tests. For variables directly related to medication use (blood pressure, serum lipid levels, glycated haemoglobin [HbA1c], serum glucose and testosterone levels; online Appendix 1, table 2), the statistical significance of a PTSD diagnosis as a predictor independent of medication was assessed by multiple linear regression, with relevant drug use as a fixed binary predictor (yes v no). Categorical data (CAC score strata) were compared in a Fisher exact test. Medical conditions that were not presented by any participants were excluded from analysis. All tests were two-tailed; to correct for multiple testing at this top-level comparison, statistical significance levels were adjusted with the Benjamini–Hochberg method.13
Two regression models were then developed. Model 1 used forward/backward stepwise regression, with seven potential confounders (age, body mass index [BMI], smoking pack-year history, AUDIT score, marital status, employment status, highest education level) and PTSD diagnosis offered into the model in an additive manner to test whether these factors accounted for the association between the PTSD diagnosis and individual comorbidities. Model 2 included the same set of confounders and the PTSD diagnosis, as well as DASS-21 depression and anxiety scores, to test whether symptoms of depression or anxiety accounted for the association between the PTSD diagnosis and individual comorbidities.
All analyses were performed in R 3.1.3 (R Foundation for Statistical Computing).
Ethics approval
Ethics approval was obtained from the Greenslopes Research and Ethics Committee (reference, 13/53), the Department of Veterans’ Affairs (reference, 014/002), the University of Queensland (reference, 2014000174), and the Queensland University of Technology (reference, 9339361).
Results
Participant characteristics
A total of 311 participants were enrolled, of whom 298 underwent all assessments (Box 1). Of these, 108 were confirmed by both psychiatric interview and CAPS-5 evaluation as having or having had PTSD, while 106 trauma-exposed controls had not, according to both measures. Participants who did not meet DSM-5 criteria for trauma exposure were excluded, as were those for whom the psychiatrist and CAPS-5 diagnoses were discordant.
The participants ranged in age from 60 to 88 years, with no significant difference between the two groups. Nor was there a significant difference in their mean weight, but mean BMI was significantly higher in the PTSD group, although the difference was small (control group, 29.0 kg/m2; PTSD group, 30.3 kg/m2; P = 0.037). More participants with PTSD had smoked recently and the group had a higher mean pack-year history. Fewer in this group currently consumed alcohol; total AUDIT scores were not significantly different, but more participants with PTSD were categorised as drinking at a risky or hazardous level or higher, indicating possible alcohol dependence. Fewer participants with PTSD were still in full-time work or had completed a university degree (Box 2).
Clinical outcomes
Direct comparison
The prevalence rates of 16 of 88 adverse outcomes were significantly higher among participants with PTSD, while the prevalence of one (history of squamous cell carcinoma or basal cell carcinoma) was lower. There were no significant differences in the reported histories of disorders affecting the endocrine or immune systems, the kidneys, or the eyes (Box 3, A; online Appendix 2, A). Participants with PTSD had a significantly higher mean number of comorbidities than those without (17.7 [SD, 6.1] v 14.1 [SD, 5.2]; P < 0.001). No participants from either group had mesothelioma, lung cancer, liver cancer, fibrosis, or cirrhosis, parathyroid problems, pituitary gland tumours, adrenal insufficiency or adrenal hormone excess, Addison disease, Conn or Cushing syndromes, phaeochromocytoma, or Parkinson disease.
The group mean values for nine of 66 clinical variables also differed significantly between the two groups; the differences were adverse for the PTSD participants, with the exception of basophil numbers. There were no significant differences in CAC scores (Box 3, B; online Appendix 2, B). No participants from either group were hepatitis B- or C- positive or had anti-mitochondrial antibodies.
After adjusting for multiple testing, five of the 24 adverse outcomes were significant predictors of PTSD after applying a 10% false discovery rate (FDR): a self-reported history of gastroesophageal reflux or peptic ulcers, and three sleep-related disorders (history of diagnosed OSA; high risk category for OSA on the Berlin Questionnaire; restless legs syndrome) (Box 3, A).
Regression analyses
After direct comparison of groups, regression models were developed for 23 of the 24 nominally significant adverse outcomes (unadjusted P < 0.05). History of seizures or epilepsy was excluded from the analysis, as no control participants reported this outcome.
Box 4 shows the ORs and 95% confidence intervals (CIs) for the relationship between PTSD and each outcome, adjusted for significant confounding demographic and risk factors (Model 1). PTSD remained significantly associated with 17 outcomes: history of MI, wheeze, gastrointestinal problems (reflux, peptic ulcers, irritable bowel syndrome [IBS], constipation), abnormal liver texture, sleep disorders (diagnosed OSA, high risk of OSA on Berlin Questionnaire, restless legs syndrome, ESS), headaches, hearing loss, forced expiratory volume ratio [FEV1%] predicted), anion gap, α1-antitrypsin levels, and caeruloplasmin levels. Seven outcomes were accounted for by the demographic and risk factors of age, BMI, smoking, and alcohol use: shortness of breath on exertion, history of fatty liver, urticaria, reduced forced vital capacity ratio (FVC%) predicted, estimated glomerular filtration rate (eGFR), triglyceride levels, and high density lipoprotein levels.
After also exploring depression and anxiety symptoms in addition to demographic confounders (Model 2), seven associations with PTSD identified in Model 1 were accounted for by symptoms of depression (history of MI, wheeze, reflux, OSA and high risk of OSA, restless legs, and ESS), and four by anxiety symptoms (abnormal liver texture, headaches, α1-antitrypsin levels, caeruloplasmin levels). Peptic ulcers, IBS, constipation, hearing loss, FEV1% predicted, and anion gap were significantly and positively associated with a PTSD diagnosis, independently of symptoms of depression and anxiety.
Discussion
We found a greater prevalence of comorbidities in Australian Vietnam War veterans with PTSD than among trauma-exposed control persons without PTSD. In particular, veterans with PTSD had higher rates of comorbidities of the gastrointestinal, cardiovascular, and respiratory systems, as well as a higher prevalence of sleep disorders. The proportions of participants with PTSD with risk factors for physical disease (higher BMI, smoking, and alcohol dependence) were greater. This is consistent with reports of associations between PTSD and obesity, lack of exercise,14 and health risk behaviours, such as smoking and alcohol dependence.12 These factors accounted for several differences between the groups, including shortness of breath on exertion, fatty liver, urticaria, eGFR, and triglyceride levels, highlighting the importance of treating these health risk behaviours as part of PTSD therapy.
The association between peptic ulcers and IBS with depression and anxiety, as well as with PTSD, was first proposed in the early 1990s.15 Case–control studies that examined the prevalence of anxiety and depression in people with functional gastrointestinal disorders have confirmed an association,16 and treating depression has been shown to improve IBS symptoms.17 The results of case–control studies investigating links between IBS and PTSD or trauma have been less clear;18–20 they have not confirmed an association between IBS and PTSD independent of trauma exposure. We found that PTSD was significantly associated with a previous diagnosis of IBS or peptic ulcer, and with a history of constipation, and that these associations were independent of depression or anxiety.
These findings highlight a major strength of our study: the rigorous diagnosis of PTSD with multiple measures for assessing trauma history, allowing us to delineate a trauma-exposed control group. Use of questionnaires with checklists for self-reported data, standardised tools, and objective clinical measures administered by clinicians helped overcome problems of recall bias, including the possibility that people with PTSD may over-report symptoms.21
The relationship between PTSD and CVD is well documented, and recent studies have found this association remains after adjusting for comorbid depression.6,10,22 We found an increased risk of MI in those with PTSD, an association explained by symptoms of depression. This is consistent with a large study22 that found the 8-year cumulative incidence of MI in people with PTSD to be 8.0%, compared with 5.9% for those who did not have PTSD; this result was also accounted for by depression.22 In contrast to other studies,23 we found no significant difference in CAC scores, suggesting that the higher prevalence of MI among PTSD patients is not explained by increased atherosclerotic plaque burden, but rather by increased susceptibility to plaque rupture and subsequent intraluminal thrombus formation, two processes associated with systemic inflammation,24 also more common in people with PTSD.
The lower predicted FEV1% in our participants with PTSD is consistent with the findings of a German report,8 but we are the first to report this finding for a veteran population in a study with a trauma-exposed control group. As in the German report, we did not observe a consistent pattern across all respiratory measures; however, chronic airway inflammation causing airflow obstruction would explain our findings.
Comorbid depression is very common in PTSD (with a prevalence as high as 50%25). We found that 22% of our participants with PTSD had depressive symptoms of moderate or greater degree according to the DASS-21. These symptoms increase the complexity and difficulty of treating PTSD, augmenting the risk that PTSD will be persistent and chronic. We also explored whether anxiety symptoms accounted for the association of PTSD with comorbid physical illness, but such symptoms should not be conceptualised as confounders, but rather as components of the symptomatology of PTSD. Symptoms shared by PTSD and anxiety disorders are treated in the same manner, including with psychological and pharmacological therapies.
Limitations to our study include its cross-sectional design, which prohibited assessing the temporal relationship between PTSD, confounders, and comorbidities. It also did not allow consideration of the time course or chronicity of PTSD symptoms. Further, the number of participants was relatively small; a larger number may have afforded the power to detect additional significant associations. Finally, it has been shown that PTSD is a risk factor for all-cause mortality among veterans of the Vietnam and other wars, independent of conventional physical comorbidities.6,23 As we studied our participants four decades after the war, they probably constituted a healthier survivor cohort than would have been observed closer to the conflict. Further, veterans needed to be well enough to participate, so that those with very severe, acute PTSD were not recruited for the study. The lack of comorbidity data from these more seriously affected veterans may have led to underestimating the extent of associations, or indeed to missing associations that contribute to early mortality in this group.
Nevertheless, our study adds further evidence about the association between PTSD and general disease. The significant contribution to physical pathology by conditions associated with PTSD (depression, obesity, alcohol and nicotine use) should alert the clinician to the importance of treating these conditions in combat veterans. Additionally, effective treatment of anxiety may significantly reduce the risk of increased physical morbidity in combat veterans. Large scale, long term prospective studies of young veterans could help elucidate the time course of disease development.
Conclusion
The higher frequency of comorbid physical conditions suggests that PTSD be conceptualised not as a purely mental disorder, but rather as a systemic disorder. Integrated health care strategies directed at the psychological and physical health of patients with PTSD, as well as rigorous control of risk factors, are likely to improve their quality of life and their survival.
Box 1 –
Study design of the Gallipoli Medical Research Institute Post-traumatic Stress Disorder Study
Box 2 –
Characteristics of the Gallipoli Medical Research Institute Post-traumatic Stress Disorder (PTSD) Study participants, by PTSD diagnosis*
|
Characteristic
|
No PTSD
|
PTSD
|
P
|
|
|
Number of participants
|
106
|
108
|
|
|
Age (years), mean (SD); range
|
69.2 (4.2); 63–86
|
68.5 (4.1); 60–88
|
0.19
|
|
Weight (kg), mean (SD); range
|
90.6 (14.1); 62–126
|
93.7 (16.2); 63–146
|
0.16†
|
|
Body mass index (kg/m2), mean (SD); range
|
29.0 (4.3); 20.6–43.0
|
30.3 (4.9); 21.2–45.8
|
0.037
|
|
Body mass index category
|
|
|
0.22
|
|
< 25 kg/m2
|
17 (16%)
|
12 (11%)
|
|
|
25–30 kg/m2
|
50 (47%)
|
44 (41%)
|
|
|
> 30 kg/m2
|
39 (37%)
|
52 (48%)
|
|
|
Smoking status
|
|
|
|
Current smoker
|
7 (7%)
|
14 (13%)
|
0.17
|
|
Recent smoker (within 12 months)
|
8 (8%)
|
21 (19%)
|
0.016
|
|
Past smoker (> 12 months ago)
|
80 (76%)
|
89 (82%)
|
0.24
|
|
Smoking pack-year history, mean (SD); range
|
18.7 (22.5); 0–105
|
26.2 (29.2); 0–150
|
0.041†
|
|
Currently consumes alcohol
|
102 (96%)
|
92 (85%)
|
0.008
|
|
AUDIT score, mean (SD); range
|
6.2 (4.3); 0–23
|
8.5 (6.8); 0–30
|
0.14†
|
|
AUDIT score category
|
|
|
0.010
|
|
Low risk (0–7)
|
74 (70%)
|
55 (51%)
|
|
|
Risky or hazardous level (8–15)
|
28 (26%)
|
38 (35%)
|
|
|
High risk or harmful level (16–19)
|
3 (3%)
|
8 (7%)
|
|
|
High risk; probably dependent (≥ 20)
|
1 (1%)
|
7 (6%)
|
|
|
Ethnic background
|
|
1.0
|
|
European
|
103 (97%)
|
102 (94%)
|
|
|
Indigenous Australian
|
0
|
1 (1%)
|
|
|
Other
|
0
|
1 (1%)
|
|
|
Not reported
|
3 (3%)
|
4 (4%)
|
|
|
Marital status
|
|
0.99
|
|
Single
|
1 (1%)
|
1 (1%)
|
|
|
Married
|
93 (88%)
|
95 (88%)
|
|
|
Divorced
|
9 (8%)
|
9 (8%)
|
|
|
Widowed
|
2 (2%)
|
1 (1%)
|
|
|
Other
|
1 (1%)
|
2 (2%)
|
|
|
Employment status
|
|
< 0.001
|
|
Full-time
|
16 (15%)
|
2 (2%)
|
|
|
Part-time
|
10 (9%)
|
7 (6%)
|
|
|
Retired
|
75 (71%)
|
76 (70%)
|
|
|
Not working
|
4 (4%)
|
22 (20%)
|
|
|
Other
|
1 (1%)
|
1 (1%)
|
|
|
Highest education level
|
|
0.006
|
|
University
|
44 (42%)
|
23 (21%)
|
|
|
Year 11 or 12
|
26 (24%)
|
24 (22%)
|
|
|
Year 10
|
14 (13%)
|
21 (20%)
|
|
|
Less than year 10
|
6 (6%)
|
16 (15%)
|
|
|
Vocational
|
16 (15%)
|
24 (22%)
|
|
|
|
AUDIT = Alcohol Use Disorders Identification Test. * Expressed as number of participants (percentage of group) unless otherwise stated. † Comparison of log-transformed distributions.
|
Box 3 –
Clinical measures for participants for which a statistically significant difference between the post-traumatic stress disorder (PTSD) and control groups was detected*
A. Binary variables: expressed as number of participants (percentage of group)
|
Outcome
|
No PTSD
|
PTSD
|
Odds ratio (95% CI)
|
P
|
False discovery rate
|
|
|
Number of participants
|
106
|
108
|
|
|
|
|
Self-reported history
|
|
|
|
|
|
|
Cardiovascular
|
|
|
|
|
|
|
Myocardial infarction
|
4 (4%)
|
16 (15%)
|
4.43 (1.43–13.7)
|
0.006
|
0.12
|
|
Cardiorespiratory
|
|
|
|
|
|
|
Symptomatic wheeze
|
15 (14%)
|
27 (25%)
|
2.02 (1.01–4.07)
|
0.046
|
0.28
|
|
Shortness of breath when exercising
|
18 (17%)
|
34 (32%)
|
2.25 (1.17–4.30)
|
0.014
|
0.17
|
|
Cancer
|
|
|
|
|
|
|
Basal cell carcinoma or squamous cell carcinoma
|
59 (56%)
|
45 (42%)
|
0.57 (0.33–0.98)
|
0.041
|
0.27
|
|
Liver
|
|
|
|
|
|
|
Fatty liver
|
4 (4%)
|
15 (14%)
|
4.11 (1.32–12.8)
|
0.01
|
0.16
|
|
Gastrointestinal
|
|
|
|
|
|
|
Diagnosed gastroesophageal reflux
|
49 (46%)
|
74 (68%)
|
2.53 (1.45–4.42)
|
0.001
|
0.048
|
|
History of peptic ulcers
|
12 (11%)
|
31 (29%)
|
3.15 (1.52–6.55)
|
0.002
|
0.048
|
|
Irritable bowel syndrome
|
12 (11%)
|
26 (24%)
|
2.48 (1.18–5.23)
|
0.015
|
0.17
|
|
Constipation
|
1 (1%)
|
8 (7%)
|
8.40 (1.03–68.4)
|
0.019
|
0.17
|
|
Neurological
|
|
|
|
|
|
|
History of any seizures (including epilepsy)
|
0
|
4 (4 %)
|
NA
|
0.046
|
0.28
|
|
Headaches (any type)
|
21 (20%)
|
35 (32%)
|
1.94 (1.04–3.63)
|
0.037
|
0.27
|
|
Sleep
|
|
|
|
|
|
|
Diagnosed obstructive sleep apnoea
|
22 (21%)
|
46 (43%)
|
2.83 (1.55–5.19)
|
< 0.001
|
0.048
|
|
Obstructive sleep apnoea, high risk Berlin category
|
44 (42%)
|
72 (67%)
|
2.82 (1.62–4.91)
|
< 0.001
|
0.035
|
|
Restless legs
|
27 (26%)
|
50 (46%)
|
2.52 (1.42–4.50)
|
0.002
|
0.048
|
|
Skin
|
|
|
|
|
|
|
Urticaria
|
10 (9%)
|
21 (19%)
|
2.32 (1.03–5.19)
|
0.038
|
0.27
|
|
Ears/hearing
|
|
|
|
|
|
|
Hearing loss
|
64 (60%)
|
82 (76%)
|
2.07 (1.15–3.73)
|
0.015
|
0.17
|
|
Measured parameters
|
|
|
|
|
|
|
Abdominal ultrasound
|
|
|
|
|
|
|
Abnormal liver texture
|
17 (16%)
|
34 (32%)
|
2.38 (1.23–4.60)
|
0.009
|
0.16
|
|
|
|
B. Continuous variables: expressed as mean (SD)
|
Outcome
|
No PTSD
|
PTSD
|
Difference of means (95% CI)
|
P
|
False discovery rate
|
|
|
Number of participants
|
106
|
108
|
|
|
|
|
Respiratory system
|
|
|
|
|
|
|
FEV1% predicted
|
87.1% (16.8)
|
81.8% (16.8)
|
–5.35 (–9.89 to –0.80)
|
0.021
|
0.18
|
|
FVC% predicted
|
94.5% (14.3)
|
89.6% (15.8)
|
–4.93 (–8.99 to –0.87)
|
0.018
|
0.17
|
|
Pathology
|
|
|
|
|
|
|
Anion gap (mmol/L)
|
7.96 (1.78)
|
8.64 (2.33)
|
0.68 (0.118–1.24)
|
0.018
|
0.17
|
|
Estimated glomerular filtration rate (mL/min/1.73 m2)
|
78.3 (13.7)
|
75 (14.5)
|
–3.27 (–7.08 to 0.533)
|
0.027§
|
0.21
|
|
Triglycerides (mmol/L)
|
1.38 (0.575)
|
1.66 (1.16)
|
0.281† (0.035–0.528)
|
0.026‡
|
0.21
|
|
Basophils (106/L)
|
39.7 (25.2)
|
33.5 (19.8)
|
–6.09‡ (0.99–1.00)
|
0.047‡
|
0.28
|
|
α1-Antitrypsin (g/L)
|
1.24 (0.20)
|
1.31 (0.22)
|
0.07 (0.01–0.13)
|
0.016
|
0.17
|
|
Caeruloplasmin (g/L)
|
0.233 (0.032)
|
0.248 (0.041)
|
0.015 (0.005–0.025)
|
0.003
|
0.072
|
|
Sleep
|
|
|
|
|
|
|
Epworth sleepiness score
|
7.6 (4.0)
|
9.2 (5.7)
|
1.6 (0.3–2.9)
|
0.018
|
0.17
|
|
|
FEV1 = forced expiratory volume in one second; FVC = forced vital capacity; NA = not applicable. * The complete data for all clinical measures are included in online Appendix 2. † Accounts for confounding medications (see online Appendix 1, table 2). ‡ Comparison of log-transformed distributions, with 95% CI = ratio of log-transformed means. § Non-parametric test (Mann–Whitney). False discovery rate was calculated with consideration of all tests performed.
|
Box 4 –
Evaluation of a post-traumatic stress disorder (PTSD) diagnosis as a risk factor for adverse outcomes. Model 1: includes significant confounding demographic factors. Model 2: evaluation of symptoms of depression and anxiety as explanatory risk factors for association of PTSD with outcomes, including significant confounding demographic factors*
A. Logistic model
|
Outcome
|
Model 1
|
Model 2
|
|
PTSD and demographic factors
|
PTSD odds ratio (95% CI)
|
Psychological and demographic factors
|
PTSD odds ratio (95% CI)
|
|
|
Heart attack/myocardial infarction
|
PTSD + smoking
|
3.74 (1.18–11.9)
|
Depression + smoking
|
—
|
|
Shortness of breath when exercising
|
Age + BMI + alcohol
|
—
|
Anxiety + age + BMI + alcohol
|
—
|
|
Wheeze
|
PTSD
|
2.02 (1.01–4.07)
|
Depression
|
—
|
|
Fatty liver
|
BMI + alcohol
|
—
|
BMI + alcohol
|
—
|
|
Reflux
|
PTSD + alcohol
|
2.25 (1.28–3.98)
|
Depression + alcohol
|
—
|
|
Peptic ulcers
|
PTSD
|
3.15 (1.52–6.55)
|
PTSD
|
3.15 (1.52–6.55)
|
|
Irritable bowel syndrome
|
PTSD
|
2.48 (1.18–5.23)
|
PTSD
|
2.48 (1.18–5.23)
|
|
Constipation
|
PTSD
|
8.4 (1.03–68.4)
|
PTSD
|
8.4 (1.03–68.4)
|
|
Abnormal liver texture
|
PTSD + BMI
|
2.09 (1.06–4.15)
|
Anxiety + BMI
|
—
|
|
Diagnosed obstructive sleep apnoea
|
PTSD + age + BMI
|
2.84 (1.47–5.48)
|
Depression + age + BMI
|
—
|
|
Obstructive sleep apnoea, high risk Berlin category
|
PTSD + BMI + alcohol
|
2.25 (1.22–4.16)
|
Depression + BMI + alcohol
|
—
|
|
Restless legs
|
PTSD
|
2.52 (1.42–4.50)
|
Depression
|
—
|
|
Headaches
|
PTSD
|
1.94 (1.04–3.63)
|
Anxiety
|
—
|
|
Urticaria
|
Alcohol
|
—
|
Alcohol
|
—
|
|
Hearing loss
|
PTSD
|
2.07 (1.15–3.73)
|
PTSD
|
2.07 (1.15–3.73)
|
|
|
|
B. Linear model
|
Outcome
|
Model 1
|
Model 2
|
|
PTSD and demographics model
|
PTSD coefficient (95% CI)
|
PTSD and demographics model
|
PTSD coefficient (95% CI)
|
|
|
FEV1% predicted
|
PTSD + age + smoking
|
–4.51 (–8.81 to –0.22)
|
PTSD + age + smoking
|
–4.51 (–8.81 to –0.22)
|
|
FVC% predicted
|
BMI + smoking
|
—
|
BMI + smoking
|
—
|
|
Anion gap (mmol/L)
|
PTSD + BMI
|
0.593 (0.034–1.15)
|
PTSD + BMI
|
0.593 (0.034–1.15)
|
|
eGFR
|
Age + BMI
|
—
|
Depression + age + BMI
|
—
|
|
Triglycerides† (mmol/L)
|
BMI + smoking + alcohol
|
—
|
BMI + smoking + alcohol
|
—
|
|
α1-Antitrypsin (g/L)
|
PTSD
|
0.071 (0.014–0.128)
|
Anxiety
|
—
|
|
Caeruloplasmin (g/L)
|
PTSD
|
0.015 (0.005–0.025)
|
Anxiety
|
—
|
|
Epworth sleepiness score
|
PTSD + BMI + smoking
|
1.62 (0.30–2.94)
|
Depression
|
—
|
|
|
BMI = body mass index; eGFR = estimated glomerular filtration rate; FEV1 = forced expiratory volume in one second; FVC = forced vital capacity. ∗ See online Appendix 1, tables 3 and 4, for complete record of analyses. † Model accounts for lipid-lowering agents as well as other confounders.
|

 more_vert
more_vert