This is a republished version of an article previously published in MJA Open
A white paper resulting from the outcomes of the Osteoporosis Australia Summit, 20 October 2011
 A PDF of the précis of this white paper that was published in print can be downloaded here.
A PDF of the précis of this white paper that was published in print can be downloaded here.
Rationale and objectives
Acronyms
|
Acronym
|
Term
|
| 1,25(OH)2D |
1,25-dihydroxyvitamin D |
| 25(OH)D |
25-hydroxyvitamin D |
| AI |
adequate intake |
| ANZBMS |
Australian and New Zealand Bone and Mineral Society |
| ANZSGM |
Australian and New Zealand Society for Geriatric Medicine |
| BMC |
bone mineral content |
| BMD |
bone mineral density |
| BMI |
body mass index |
| CI |
confidence interval |
| DEQAS |
UK Vitamin D External Quality Assessment Scheme |
| DRI |
dietary reference intake |
| DXA |
dual energy x-ray absorptiometry |
| EAR |
estimated average requirement |
| ESA |
Endocrine Society of Australia |
| HPLC |
high-performance liquid chromatography |
| HSA |
hip structural analysis |
| IOM |
US Institute of Medicine |
| LC-MS/MS |
liquid chromatography–tandem mass spectrometry |
| MI |
myocardial infarction (heart attack) |
| MRI |
magnetic resonance imaging |
| NHMRC |
National Health and Medical Research Council |
| NRV |
nutrient reference value |
| OA |
Osteoporosis Australia |
| PBS |
Pharmaceutical Benefits Scheme |
| pQCT |
peripheral quantitative computed tomography |
| PTH |
parathyroid hormone |
| PTHrP |
parathyroid hormone-related protein |
| QCT |
quantitative computed tomography |
| RACF |
residential aged care facility |
| RCT |
randomised controlled trial |
| RDA |
recommended dietary allowance |
| RDI |
recommended dietary intake |
| SRM |
standard reference material |
| UL |
upper level of intake (Australia) or tolerable upper intake level (US) |
| vBMD |
volumetric bone mineral density |
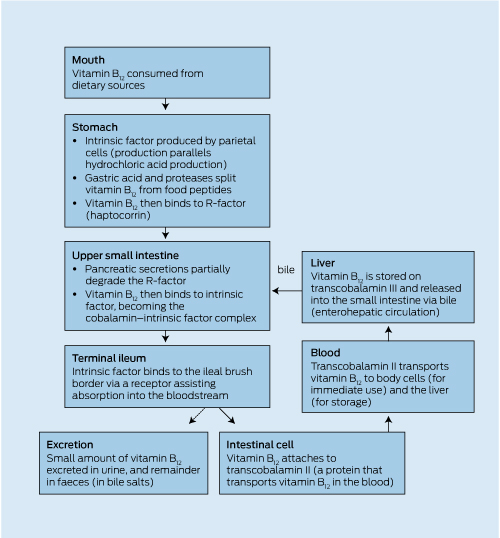
Both general practitioners and their patients often overlook bone health and, as a result, osteoporosis is often not diagnosed until fragility fractures occur. There is also a lack of an accepted strategy for osteoporosis prevention in Australia. Currently, treatment of individuals is based on either bone mineral density (BMD; see Box of acronyms) measurement and/or a prior fracture. However, more than 50% of women and 70% of men who sustain fragility fractures do not have BMD in the osteoporosis range (T score < − 2.5).1 This represents a “prevention paradox”, which is the basis for developing this population-based prevention strategy throughout the life cycle, Building healthy bones throughout life.
For many individuals, taking simple preventive actions throughout life will enable them to continue to enjoy the active and independent lifestyle that is associated with bone health. The aim of the 2011 Osteoporosis Australia Summit, Building healthy bones throughout life, was to develop clear, succinct, evidence-informed recommendations about calcium, vitamin D and exercise requirements for building healthy bones in children and adolescents, healthy adults and older adults.
The 2011 Summit brought together a multidisciplinary group of about 120 experts, including bone specialists, GPs, researchers, nutritionists, research nurses, physiotherapists, exercise specialists, consumer representatives, peak bodies, and state and federal government policymakers. This event provided an important opportunity to review the latest data and debate the current issues relating to bone health and the prevention of osteoporosis, which affects 1.2 million Australians.
We invited experts in particular fields to contribute short articles that were incorporated into a draft white paper. The draft white paper was available for public comment until 30 September 2011. The revised and penultimate draft paper was put forward for further discussion and consensus at the 2011 Summit, and this current paper is the final result.
Osteoporosis Australia will ensure the outcomes of the 2011 Summit and this associated Building healthy bones throughout life paper are widely disseminated to consumers and medical and health care professionals.
Overview
Building healthy bones throughout life provides the evidence for three affordable and important ways of reducing the enormous personal and economic costs of osteoporosis — a major cause of pain, disability and premature death affecting both women and men. The three interventions are ensuring people have (1) an adequate level of calcium, (2) an adequate level of vitamin D, and (3) appropriate physical activity throughout their lives.
International and national research leaders in bone health prepared this paper. They reviewed the science and formulated evidence-informed recommendations, which have also been reviewed by relevant stakeholder groups and the public. Building healthy bones throughout life does not resile from the recent debates about whether calcium supplements increase the risk of cardiovascular events and uncertainty about the optimal levels of serum vitamin D for bone health. The recommendations in this paper balance the risks — if they truly exist — with the benefits of preventing and slowing the onset of osteoporosis.
The relative importance of these three interventions and the need for supplementation changes throughout life; therefore the recommendations in this paper on the roles of calcium, vitamin D and weight-bearing exercise on bone health are separated into categories for children, adults, and older adults, as well as individuals with osteoporosis. The core message is that adequate dietary calcium and optimal vitamin D intake, together with regular weight-bearing exercise and moderate sunlight exposure are important at all stages of life in healthy individuals.
The implementation of this paper’s recommendations is likely to significantly reduce the direct and indirect costs of osteoporosis, which currently affects 1.2 million Australians, not to mention the 6.3 million people in this country with low bone density (osteopenia).2 Addressing the calcium, vitamin D and physical activity levels required for bone health will require the close collaboration and engagement of health services, governments, insurers, clinicians and consumers in a range of innovative strategies.
Executive summary
Osteoporosis currently affects 1.2 million Australians, most of whom don’t know they have the disease.2 Without intervention, this number is expected to increase to 3 million by 2021 as a result of population ageing.3 In addition, there are now 6.3 million Australians with thin bones (osteopenia).2
Risk factors
Some factors that increase the risk of osteoporosis cannot be changed; these non-modifiable factors include being female (women develop thin bones sooner than men), menopause, age, certain medical conditions, and a genetic predisposition. However, there are a number of readily modifiable risk factors that can be changed to reduce the risk of osteoporosis. These include:
- lack of weight-bearing exercise
- poor calcium intake
- vitamin D deficiency (serum 25-hydroxyvitamin D [25(OH)D] level < 50 nmol/L, measured in late winter/early spring)
- low or high body weight
- cigarette smoking
- excessive alcohol use
- long-term use of corticosteroids.
It is known that if we are able to reduce the burden of fractures caused by osteoporosis by around 20%, it would significantly reduce both the direct costs of health care and the indirect costs to families and the economy. Even if just calcium intake and vitamin D levels were addressed, the direct costs of osteoporosis in Australia could be lowered by up to $432 million per year.3
Calcium
The median dietary intake of calcium in the last Australian National Nutrition Survey was 827 mg per day for men and 663 mg per day for women;4 this indicates that men’s calcium intake was close to the estimated average requirement (EAR) of 840 mg per day, but women were falling well short of the EAR.
Most systematic reviews of the scientific evidence favour supplementation of calcium plus vitamin D to reduce fracture risk, with an overall benefit of 10%–20% compared with placebo, while even greater reductions in fracture risk (≥ 30%) have been observed in the elderly and the institutionalised. The combination of vitamin D with calcium has also been shown to reduce mortality by 7%.
Calcium needs in children
The aim with calcium intake in children and adolescents is to optimise their peak bone mass (their “bone in the bank”). During infancy, calcium provided by breast milk is assumed to be adequate and is the basis for most recommended intakes in the first few months of life. However, findings from the 2007 Australian National Children’s Nutrition and Physical Activity Survey5 indicated that girls aged 12–16 years and boys aged 14–16 years appeared to be most at risk of not meeting their daily calcium dietary requirements (82%–89% of girls did not meet the EAR of 1050 mg/day; 44% of boys did not meet the EAR). It was suggested that this is likely due to a substantial decline in milk intake in childhood and its replacement with carbonated beverages.
Systematic reviews of randomised controlled trials (RCTs) of calcium supplements in children6 suggest that increasing calcium intake from an average 700 mg per day to 1200 mg per day has limited benefits for bone mass. The evidence therefore does not support the use of calcium supplements in healthy children with the possible exception of those with very low intakes (< 700 mg per day). Even so, calcium intakes in Australian children are often inadequate, so there is still a challenge to ensure healthy nutrition and good levels of weight-bearing physical activity to build peak bone mass.
Calcium needs in healthy adults
There are only limited studies into the role of calcium intake in maintaining bone mass or fracture prevention in young and middle-aged adults.
An adequate calcium intake achieved through diet continues to be the best choice in those who can include an adequate intake of dairy products. In practice, this translates to 3–4 servings of calcium-containing foods each day. To achieve the recommended intakes of 1000–1300 mg per day, most adults would routinely need to also include at least one serving of a calcium-fortified food.
There is no evidence that individuals consuming calcium at levels significantly higher than the allowance receive any additional skeletal benefit.7,8
Calcium needs in postmenopausal women and the elderly
There is evidence that the calcium needs in this group are greater and nutrition can also be more precarious, which means supplementation is a key strategy to ensure adequate calcium (and vitamin D) levels. Systematic reviews of RCTs aimed at preventing fractures show calcium can help prevent osteoporosis and fragility fractures.9,10,11 Nevertheless, there is significant variation in the results12,13,14,15 because of differences in dosage, baseline nutrient status, and the co-administration of vitamin D, as well as poor adherence to the supplements.
The frail elderly have the highest rates of fracture and those in residential care typically have vitamin D deficiency and an inadequate calcium intake, which means that these people have the greatest potential to benefit from increased intake of calcium alone or calcium plus vitamin D. The evidence is mixed but, on balance, shows that calcium supplementation prevents fractures in the frail elderly, particularly in women in residential care.11
There have been well designed, randomised trials of calcium supplementation that have reported no significant effect on fracture prevention using intention-to-treat analysis.11,13,15,16 However, this has been explained by high rates of poor adherence to the supplements (55%–60%) and when some analyses have been performed keeping faith with the original intent of the trials (“per protocol” analysis), benefit has been shown.14,17
Recent re-analyses of adverse events from some trials have suggested the use of calcium supplements may be associated with an increased risk of cardiovascular events.18 These claims of increased heart attack (or myocardial infarction [MI]) risk have generated considerable scientific debate and a re-evaluation of the risk–benefit analysis of calcium supplementation. The conclusion of this Building healthy bones throughout life paper is that, as none of the trials re-analysed were primarily designed to investigate cardiovascular outcomes,19 on balance, current evidence does demonstrate a small increase in risk of MI with calcium supplements. Using Women’s Health Initiative data, based on the worst-case scenario, treatment of 1000 people with calcium or calcium and vitamin D for 5 years would cause an additional six MIs or strokes and prevent three fractures.20 However, mortality is not increased and, in fact, the combination of vitamin D with calcium supplements has been found to reduce mortality in the elderly by 7%.21
A cohort study also shows that self-reported calcium supplement use was associated with a 9% reduction in mortality in older women.22 If calcium and vitamin D are taken more than 80% of the time, the prevalence of fractures has been found to decrease by 24% in older men and women.11
Calcium or calcium–vitamin D supplements may be beneficial for general health as well as reducing fracture risk in people who may not be getting enough calcium through their diet.21 Nevertheless, dietary calcium is the preferred source of calcium, and calcium supplements should be limited to 500–600 mg per day.
Vitamin D
Vitamin D adequacy is important for bone and muscle function. Vitamin D deficiency is common in Australia. The findings from a national population-based sample of 11 218 Australian adults aged 25–95 years showed that 31% of the population had a serum 25(OH)D level < 50 nmol/L, and that the prevalence of deficiency increased with age, and was greater in women compared with men, in those of non-European ancestry, and in those living in the southern states of Australia, particularly during winter.23 For instance, in people residing in southern Australia (latitude > 35°S), 42% of women and 27% of men had 25(OH)D levels < 50 nmol/L during summer and autumn, which increased to 58% of women and 35% of men during winter and spring.23 These findings are consistent with an earlier study, which combined results from mostly normal populations in south-east Queensland, Victoria and Tasmania, and reported vitamin D insufficiency (defined as < 50 nmol/L) during winter and spring in 40% of women in Queensland, 37% of women in Victoria and 67% in Tasmania.24 Similarly, the most recent population-based study of 3653 rural and urban Victorians sampled from May 2009 to April 2010 showed 44.1% of both rural and urban-dwelling Victorians had a serum 25(OH)D level < 50 nmol/L.25
Older people, particularly those living in residential aged care facilities, are at high risk of vitamin D deficiency.26,27,28 Others at greatly increased risk of vitamin D deficiency include: people with dark skin;29,30,31 those who wear modest dress, which covers most of their bodies;29,30,32 people at high risk of skin cancer either because of past history or due to immunosuppression;33,34,35 people with intestinal malabsorption of key nutrients;36 people less likely to spend time in the sun, including those with chronic diseases, transplant recipients, people taking antiepileptic medications,37,38 office workers and shift workers.35
Sunscreen use in the general population is not associated with low vitamin D levels, despite sunscreen’s theoretical capacity to block most UVB.32,35,39 Inadequate application combined with higher sun exposure in people who use sunscreen probably explains the discrepancy.
Vitamin D needs in pregnancy and childhood
Recent Australian research has confirmed the high prevalence of low vitamin D levels in pregnant women,40 and the potential adverse effects on fetal bone health and other pregnancy outcomes. As such, it is reasonable to check vitamin D status in all pregnant women and supplement to achieve maternal levels > 50 nmol/L,41 although the question of whether empirical supplementation without testing would be cost-effective remains unanswered.
Vitamin D is important for bone health and muscle function throughout childhood and adolescence. Adequate vitamin D status is required to prevent rickets and to promote normal bone growth and mineralisation as peak bone mass is acquired. Based on available evidence, the recommended blood level of 25(OH)D for infants, children and adolescents for optimal bone health remains at > 50 nmol/L year round.
Vitamin D needs in healthy adults, older adults and individuals with osteoporosis
Both the recent position statement on vitamin D and health in adults from the Australian and New Zealand Bone and Mineral Society (ANZBMS), the Endocrine Society of Australia (ESA) and Osteoporosis Australia (OA)42 and the recent US Institute of Medicine (IOM) report on dietary reference intakes for calcium and vitamin D concluded that inadequate vitamin D status is defined as a 25(OH)D level < 50 nmol/L at the end of winter.43 Levels of 25(OH)D may need to be 10–20 nmol/L higher at the end of summer to allow for seasonal variation. The IOM report used a 25(OH)D level of 50 nmol/L to determine the recommended dietary allowance for vitamin D.43 Systematic reviews of trials of vitamin D supplementation to prevent falls and fractures have found serum 25(OH)D thresholds should be 60 nmol/L and 75 nmol/L, respectively.44,45 A recent Endocrine Society clinical practice guideline recommends that adults aged 50–70 years and those over 70 years require at least 600 IU and 800 IU (15 µg and 20 µg) of vitamin D3 per day, respectively, to maximise bone health and muscle function.46 However, to raise the serum level of 25(OH)D > 75 nmol/L, as both the Endocrine Society and the International Osteoporosis Foundation47 recommend, may require at least 1500–2000 IU (37.5–50 µg) per day of supplemental vitamin D, while for severe deficiency, doses ≤ 10 000 IU (250 µg) per day have proven to be safe.46
Other nutritional influences
There is considerable evidence of the positive influence that dietary patterns adequate in calcium, phosphorus and vitamin D have on bone health.48 However, less consistent evidence exists on the role of other vitamins and micronutrients. A large-scale study from the US showed dietary patterns that included high intakes of vegetables and fruit were associated with significantly higher bone mineral density (BMD) than those with other dietary patterns.49 Therefore, it seems prudent to encourage a variety of foods, particularly fruits and vegetables, to ensure adequate intakes of key nutrients to maintain bone health.
Exercise
Regular physical activity and exercise is recognised as one of the most effective lifestyle strategies to maximise peak bone mass during growth and prevent bone loss during ageing. However, the bone-building (osteogenic) benefits of exercise are dependent on stage of life and the relative risk of fracture. Childhood and adolescence may represent the optimal window of opportunity in which exercise can improve bone strength and protect against osteoporosis and fragility fractures in old age, particularly when these early benefits can be maintained by adopting appropriate measures in later life. A 10% higher peak bone mass can delay the development of osteoporosis by 13 years and reduce the risk of fracture by 50%.50,51
Exercise programs that combine high-impact activity with high-intensity resistance training appear most effective in augmenting BMD in premenopausal women. High-impact-alone protocols (such as jumping) are effective only on hip BMD in this group.52 Further RCTs of resistance training in premenopausal women of sufficiently long duration to provide optimum resistance-training type, intensity and volume of loading are needed.53
In frail and very elderly adults, resistance training and balance exercises in combination reduce falls and risk factors for frailty, including sarcopenia, poor balance, gait instability, depression, fear of falling and cognitive impairment.
Multimodal exercise — including weight-bearing/high-impact/high-intensity resistance exercise — may significantly reduce overall fracture risk.54 By contrast, single-modality exercise of any type does not appear to reduce fracture risk,54 with the possible exception of spinal-extensor muscle resistance training, which reduces thoracic vertebral fracture incidence.54,55
Antiresorptive and anabolic agents
In individuals at high risk of fracture, especially those who have already had previous fractures, specific anti-osteoporosis therapy reduces vertebral fracture risk by 40%–70% and non-vertebral fractures by about 25%.56,57,58,59,60,61,62,63,64,65,66 The various anti-osteoporosis treatments have been separately evaluated in placebo-controlled RCTs with fracture end points; however, a corresponding head-to-head comparison has not been conducted. In Australia, these treatments are covered by the Pharmaceutical Benefits Scheme (PBS) for men and women after fragility fracture as well as for those at high risk, without prior fracture, on the basis of age (≥ 70 years) and low BMD T-score (≤ − 2.5 or ≤ − 3.0).67
Recently, it has been suggested that a temporary cessation of treatment (“drug holiday”) can be offered to patients after 3–5 years of treatment with antiresorptive therapy. Currently, there is no evidence to support a drug holiday in individuals with continuing osteoporosis (T score ≤ − 2.5 at femoral neck).68 If a drug holiday is considered appropriate, a plan must also be put in place to review the patient regularly. It seems prudent to reinstate therapy if there is any further bone density decline, which is usually preceded by an increase in bone turnover marker levels.
Bone density testing
The current ideal test to assess fracture risk is the dual energy x-ray absorptiometry (DXA) to measure lumbar spine and proximal femoral BMD in all high-risk individuals.69 In addition, the use of DXA to screen asymptomatic individuals may be worthwhile at age 65 or 70 years.70,71 Currently, Australian Medicare funds this approach in people over the age of 70.
Recommendations
Final recommendations for this paper were developed through the process of consultation, review and discussion at the Osteoporosis Australia Summit meeting on 20 October 2011.
Recommendations
Bone health is often overlooked as a serious public health problem and as a result, osteoporosis is often not diagnosed until fragility fractures occur. Based on a large population-based study of bone mineral density (BMD) measurements in Australian adults, it is estimated that 1.2 million Australians have osteoporosis and a further 6.3 million are at risk with osteopenia (low BMD).2
For many individuals, taking simple osteoporotic preventive actions with calcium, vitamin D and exercise throughout life will enable them to continue to enjoy an active and independent lifestyle that is associated with good bone health. The mandate of the 2011 Osteoporosis Australia Summit Building healthy bones throughout life was to develop clear evidence-informed recommendations about calcium, vitamin D and exercise requirements in children, healthy adults, and older adults and individuals with osteopenia and osteoporosis. The recommendations that follow describe calcium, vitamin D and exercise needs relevant to all stages of life, and also highlight specific needs during childhood, midlife and old age.
Recommendations for all stages of life
Calcium
- Eat sufficient and nutritious foods for growth and development.
- Daily dietary calcium intakes should be consistent with the Australian and New Zealand guidelines for an adequate calcium intake.4
- It is agreed that a diet low in calcium increases the risk of bone loss and fracture. The Australian and New Zealand guidelines for an adequate calcium intake are shown in Box 1.
- Calcium needs are increased during the adolescent growth spurt.
- Practically, people should aim to include 3–5 serves of calcium-rich foods daily (eg, dairy or calcium-fortified foods), as the preferred means of achieving an adequate calcium intake. Box 2 provides the calcium content of key foods.
- Individuals who dislike or are intolerant of dairy products and wish to achieve their required calcium intake from diet will need to have more serves of other high-calcium-containing foods (eg, specific vegetables, fish, nuts) or calcium-fortified foods (eg, soy milk).
- For people with inadequate dietary calcium intake (below the estimated average requirement [EAR]/recommended dietary intake [RDI]), calcium supplements are recommended and are as effective as dietary sources. Under these circumstances, calcium supplementation with 500–600 mg per day is indicated.
- Achieve and maintain a healthy body weight to maintain muscle mass, particularly guarding against underweight and overweight.
- In population studies, fracture risk is increased in females with low BMI and body fat, especially if body weight is sufficiently low to impair sex hormone production.
- Population studies also show that obesity is not protective against fractures in some individuals.72,73
- Sarcopenia is associated with low BMD and an increased risk of fracture.74,75,76,77
Vitamin D
- Ensure adequate vitamin D levels.
- Sun exposure is the primary source of vitamin D. Encourage regular and safe sunlight exposure (avoiding burning), in accordance with current Australian and New Zealand Bone and Mineral Society (ANZBMS), Endocrine Society of Australia (ESA) and Osteoporosis Australia (OA) recommendations.42 However, there is a need for more research in this area. Box 3 provides guidance on recommended sun exposure from the vitamin D position statement supported by the ANZBMS, the ESA and OA.42
- Maintaining adequate vitamin D is critical for calcium absorption and is also important for optimal bone health and muscle function.
- There is general agreement that serum levels of 25-hydroxyvitamin D (25[OH]D) in the general population should be above 50 nmol/L at the end of winter or in early spring for optimal bone health.
- Most adults will not receive more than 5%–10% of their vitamin D requirements from dietary sources. In healthy adults, the main contributor to circulating vitamin D levels is vitamin D produced in the skin in response to sunlight exposure.
- Current evidence does not support a case for food fortification with vitamin D. More evidence on whether there is widespread vitamin D deficiency is required before such a case can be supported.
- If sun exposure is limited or there are other risk factors for vitamin D deficiency (dark skin, clothing covering the skin, conditions affecting vitamin D metabolism, breastfed babies with other risk factors), it is important to measure the serum 25(OH)D level and take vitamin D supplements in doses that will maintain serum 25(OH)D levels over 50 nmol/L year round.
- For people who do not get adequate exposure to sunlight, vitamin D supplements provide a means of increasing vitamin D intake. To treat moderate to severe deficiency, it would be reasonable to use 3000–5000 IU (75–125 µg) of vitamin D supplements per day for at least 6–12 weeks, with most patients requiring ongoing treatment at a maintenance dose of around 1000–2000 IU (25–50 µg) per day. Higher doses of 2000–4000 IU (50–100 µg) per day may be required in some individuals (eg, if obese).
Exercise
- Undertake regular weight-bearing physical activity, muscle-strengthening exercises and challenging balance/mobility activities in a safe environment and promote a healthy lifestyle.
- Encourage regular participation in a variety of weight-bearing activities, including dynamic impact-loading sports (eg, basketball, netball, hockey, football, soccer), school-based physical education classes and regular outside play, for at least 30 minutes 3–5 days per week.
- For healthy individuals (without osteoporosis) with few risk factors for fracture, the key focus of exercise and physical activity is to improve or maintain bone density, muscle mass, strength and functional capacity (balance, gait). A combination of weight-bearing and resistance training is recommended.
- Some examples of the impact of particular exercises on bone health are shown in Box 4.
- For individuals with osteoporosis and/or at increased risk of falling, challenging balance and mobility exercises are recommended.
- Exercise offers greater skeletal benefits when undertaken with a diet containing an adequate intake of calcium (equivalent to the EAR/RDI).
- Avoid prolonged periods of sedentary behaviours (sitting), due to detrimental effects on bone and cardiovascular health.
Other
- Encourage health promotion models to reduce uptake of smoking, dieting behaviours and alcohol use.
- If alcohol is consumed, it should be consumed in moderation — up to one standard drink per day for women and two standard drinks per day for men.
- Excessive alcohol intake is a cause of fracture, because of an increased propensity to fall.
- Excessive alcohol also impairs bone formation.
- Do not smoke. Smoking is associated with a reduction in bone structure and strength.
- Maintain normal sex hormone levels for the stage of life, and correct levels as appropriate in premenopausal women and men.
- Test for bone health.
- Population-based vitamin D testing using a blood sample is not recommended.
- Vitamin D testing is not recommended in otherwise healthy individuals who do not have risk factors or disorders predisposing to osteoporosis and minimal trauma fracture.
- If sunlight exposure is very low or there are other risk factors for vitamin D deficiency (dark skin, absence of skin exposure), testing may be recommended. If vitamin D testing is recommended, it should be done at the end of winter or in early spring.
- Consider bone density testing, using dual energy x-ray absorptiometry, in the presence of risk factors or at age ≥ 70 years in the absence of risk factors.
Recommendations for building healthy bones in children
In addition to the above general recommendations, these recommendations are designed to provide advice to parents and carers relating to steps they can take to promote healthy bone growth in children. They are also intended to provide public health recommendations during pregnancy and lactation, childhood, and the teenage years.
Peak bone mass is acquired during late adolescence and early adulthood and sets the stage for vulnerability to fracture and other bone disorders later in life. The 2 years around puberty is a particularly important period to maintain adequate calcium and engage in weight-bearing exercise, as about 40% of adult peak bone mass is acquired during this period.78
- Ensure adequate calcium intake.
- Encourage and support breast feeding. Breast milk is an important source of calcium. Infants should be exclusively breastfed to 6 months of age and continue to be breastfed, with complementary foods, until 12 months.
- Reduced-fat milk products are not suitable for children under 2 years of age.
- Ensure adequate vitamin D.
- Promote adequate maternal vitamin D status during pregnancy.
- Breastfed babies from women at risk of vitamin D deficiency require supplementation.
- Children with chronic illness or disability warrant special consideration of their vitamin D status and bone health.
- Engage in regular weight-bearing activity and promote a healthy lifestyle.
- Encourage schools to incorporate a diverse and enjoyable battery of weight-bearing activities and sports into their school physical education programs. This could include participation in short periods (5–10 minutes) of daily, targeted, multidirectional, moderate- to high-impact activities, such as jumping, skipping and hopping.
Building healthy bones in healthy adults
These recommendations are designed to augment the general recommendations and provide specific advice to healthy adult individuals relating to steps they can take themselves to reduce the risk of fracture in the future. They also include public health recommendations for the prevention of fracture in adults who have achieved peak bone mass and are at low risk of fracture. Because fracture risk increases with age, these recommendations are particularly important for individuals who wish to maintain their bone strength into old age, and especially for postmenopausal women and older individuals to maintain their bone strength.
The needs of adults with specific disorders affecting the skeleton (eg, osteoporosis, coeliac disease) are not addressed in these recommendations; these individuals should seek specific medical advice appropriate to the condition.
- Ensure adequate vitamin D levels (see Recommendation 3 for all stages of life).
- Be habitually physically active and undertake regular weight-bearing and/or muscle-strengthening exercises.
- Encourage regular participation in moderate-impact weight-bearing physical activity, high-impact training (eg, 50–100 jumps) or related impact-loading sports for at least 30 minutes 3–5 days per week.
- Include muscle-strengthening exercises on at least 2 days per week. For maximum benefits, the program should be high intensity (60%–80% of peak capacity), become progressively more challenging over time, and target the major muscles around the hip and spine.
- Where possible, encourage participation in a multimodal exercise regimen (including weight-bearing/high-impact/high-intensity resistance exercise) at least three times per week.
Building healthy bones in older adults and individuals with osteopenia and osteoporosis
These recommendations are designed to supplement the general recommendations and provide specific advice to individuals relating to steps they can take themselves to reduce fracture risk, and to provide them with information on how best to access appropriate health advice. They are public health recommendations for the prevention of fracture in adults > 50 years of age who are at higher risk of fracture, defined as having a 5-year absolute risk of fracture over 5%.
Adults with specific disorders (such as coeliac disease or conditions for which they take oral corticosteroids) that may be responsible for their low bone density should also seek specific medical advice appropriate to the condition.
Adults with a 5-year absolute risk of fracture over 10% should also seek specific advice on the management of osteoporosis, if present, which is well covered in the Royal Australian College of General Practitioners’ Clinical guideline for the prevention and treatment of osteoporosis in postmenopausal women and older men.79
- Dietary calcium intakes should be consistent with the Australian and New Zealand guidelines for an adequate calcium intake.4
- Calcium intake by diet is strongly recommended, but calcium supplements at doses of 500–600 mg per day may be required in some individuals when calcium from dietary sources is not possible.
- Current concerns over the potential for an increased risk of myocardial infarction with calcium supplements are still being debated but should not alter acceptance of the recommendation. Mortality has not been increased in any study of calcium supplements.
- Vitamin D plays an important role in bone health.
- In addition to the general recommendations made in Recommendation 10 for all stages of life, vitamin D level should be established by measuring vitamin D levels in the blood in the following situations:
- osteoporosis when diagnosed by bone density testing;
- after falling;
- following a minimal trauma fracture.
- If 25(OH)D levels are below the desirable level, the following doses are recommended:
- supplementation with vitamin D capsules or tablets is recommended in doses of 1000–2000 IU (25–50 µg) per day;
- higher dose intermittent therapy, eg, 50 000 IU (1250 µg) per month, is an alternative, although more data on the safety of monthly dosing are required.
- The desired outcome of vitamin D supplementation is:
- a reduced risk of fractures if serum 25(OH)D levels are above 75 nmol/L;
- a reduced risk of falls if serum concentrations are above 60 nmol/L;
- individuals with serum 25(OH)D levels above 50 nmol/L at the end of winter or in early spring are likely to have levels of 60–75 nmol/L for much of the remainder of the year.
- For older adults, the elderly and those with or at risk of osteoporosis, falls and fracture, the key focus of exercise should be to not just slow bone loss, but to increase or maintain muscle mass and muscle strength, and to improve muscle function, gait and mobility to reduce the risk of falls and fractures.
- Encourage participation in a multimodal and supervised exercise program that includes weight-bearing activities, progressive resistance training and high challenging balance and functional activities at least three times per week.
- It is important that muscle groups connected to bones of relevance to osteoporotic fracture be emphasised in such programs (eg, spinal extensor muscles, hip abductors, hip extensors, knee extensors/flexors) and those related to gait and balance (ankle plantar flexors and dorsiflexors, inverters and everters, hip abductors).
- Regular leisure-time walking should be encouraged for its benefits on weight control and cardiovascular health. For skeletal health benefits, it is recommended that individuals progress to brisk or hill walking and then to other forms of moderate-impact weight-bearing exercises.
- Elderly people with osteoporosis and a history of fracture should avoid exercises or activities that involve forward flexion of the spine, particularly while carrying weights.
- Maintain safe environments to avoid falls and encourage falls education. Elderly individuals should consider and address risk factors for falls (vision problems, use of sedatives, postural hypotension, environmental hazards).
Overview of bone health in Australia
Potential economic impact if bone health is not effectively addressed
Burden of disease — osteoporosis in Australia
Osteoporosis and osteopenia affect 1.2 million and 6.3 million Australians, respectively.2 Without preventive intervention, the number of osteoporosis sufferers is expected to increase to 3 million by 2021 as the population ages.3 For people over the age of 60 years, one in two postmenopausal women and one in three older men will suffer an osteoporosis-related fracture.3 Mortality is increased after all fragility fractures, particularly hip fractures.80 Over one in four people who suffer a hip fracture will die during the first year and less than one-third will regain their prefracture level of mobility.81 Thus, the risk of mortality among women with a hip fracture is similar to or higher than that in women with breast cancer.81 Based on the 2001 Access Economics report commissioned by Osteoporosis Australia, the total direct care cost of osteoporosis is estimated to be over $1.9 billion per year in Australia, with an additional $5.6 billion expended in indirect costs.82
Doctor-reported osteoporosis and Indigenous Australians
An estimated 692 000 Australians (3.4% of the total population) had doctor-diagnosed cases of osteoporosis in 2007–08 based on data from the Australian Institute of Health and Welfare.83 Women accounted for the majority of cases (81.9%).
Although the disease occurs mainly in people aged 55 years and over (84.0%), osteoporosis is a condition without overt symptoms and is known to be underdiagnosed. While the extent of this is difficult to establish, the prevalence of doctor-diagnosed osteoporosis is almost certainly an underestimate. The diagnosis of osteoporosis is more prevalent among those who live in major cities than in rural and remote locations, but this may relate to lack of access to the diagnostic test, bone densitometry, in the latter locations.84
In 2004–05, 0.74% of Indigenous men and 1.11% of Indigenous women reported having doctor-diagnosed cases of osteoporosis. The age-standardised prevalence rates show that osteoporosis was more common among Indigenous men (1.8 times) but less common among Indigenous women (0.5 times) than in their non-Indigenous counterparts.83 Osteoporosis is not more common in those born overseas or those from a low socioeconomic class.83
The hip and pelvis (40.5%) and wrist and forearm (17.1%) were the most common sites of minimal trauma fractures in 2007–08. Interestingly, the age-related rates of hospital separations for minimal trauma hip fracture decreased between 1998–99 and 2007–08, a trend that is consistent with reports from North America and Scandinavia.83 In Australia, the age-related incidence of hip fracture decreased by 15% and 8% in women and men, respectively, between 1998–99 and 2007–08. However, the total number of minimal trauma hip fractures rose from 14 671 to 17 192 over the same period.83
Osteoporosis was managed at a rate of 1 in 100 GP–patient encounters in 2007–08, double the rate seen in 1998–99. Advice and the prescription and supply of medications were the mainstays in these encounters. In hospital settings, both surgical procedures and allied health services were provided to treat fractures.83 Osteoporotic fractures, particularly minimal trauma hip fractures, can lead to premature deaths among the elderly; mortality is increased for at least 5 years even after minor minimal trauma fractures.80
Risk factors
Some risk factors for osteoporosis are non-modifiable, such as female sex, menopause, age, other metabolic disorders, and a genetic predisposition to poor skeletal health. These are useful markers to identify people at increased risk of developing osteoporosis. However, several other risk factors for osteoporosis are readily modifiable:
- lack of weight-bearing exercise
- poor calcium intake
- vitamin D deficiency (serum 25-hydroxyvitamin D [25(OH)D] level < 50 nmol/L, measured in late winter/early spring)
- low or high body weight
- cigarette smoking
- excessive alcohol use
- long-term use of corticosteroids.3
Cost savings by implementing osteoporosis risk mitigation strategies
Although many risk factors for osteoporosis are modifiable, evidence of the skeletal benefits of risk factor mitigation is limited, with the exception of adequate calcium intake and vitamin D levels and the reduction of corticosteroid doses. The evidence for vitamin D reducing the risk of non-vertebral and hip fractures is most compelling with the use of additional calcium.11,44,85,86,87,88,89 In women and men aged > 50 years, the combination of vitamin D with calcium, but not vitamin D alone, had a modest effect in preventing fractures (relative-risk reductions of 13%–24%), particularly in those with long-term compliance rates ≥ 80%.11 According to this study, the daily dose of vitamin D and calcium should be at least 800 IU (20 µg) and 1200 mg, respectively. These values are somewhat different to those recommended in the Australian guidance for vitamin D when sun exposure is minimal, at 600 IU (15 µg) per day for those aged ≤ 70 years and 800 IU (20 µg) per day for people aged > 70 years.42 These recommendations appear conservative, and those with substantial sun avoidance may require higher doses.
Swedish data show the combination of calcium and vitamin D is cost-effective in 70-year-old women at an efficacy as low as 67% of that seen in clinical trial data. Treatment was also cost-effective in 50–60-year-old women with osteoporosis or a family history of maternal hip fracture.90 A Markov model using the efficacy rate in an important hip fracture prevention trial88,89 and prevalence data for osteoporosis in Sweden91 show the costs of treating all 70–79-year-old women and 25% of women 70 years or older in Sweden with calcium and vitamin supplementation would be offset by savings from reductions in fracture rates. Another study shows that increasing the serum 25-hydroxyvitamin D (25[OH]D) level of all Europeans to 80 nmol/L has the potential to reduce the total direct economic burden of diseases related to vitamin D deficiency by 11.4% or €105 000 million,92 depending on the effects of vitamin D on chronic diseases.
Summary
Osteoporosis affects 1.2 million Australians,2 many of whom are unaware they have the disease. Without preventive intervention, this number is expected to increase to 3 million by 2021 as the population ages.3 The total direct care cost of osteoporosis is estimated to be over $1.9 billion per year in Australia, with an additional $5.6 billion expended in indirect costs.82 While many risk factors for osteoporosis are modifiable, the evidence of the skeletal benefits of risk factor mitigation is limited. The best evidence is for adequate calcium intake and adequate vitamin D levels. Vitamin D replacement for primary fracture prevention is effective in those who have inadequate serum levels of 25(OH)D, particularly in institutionalised patients, and when combined with calcium supplements. Such a strategy of increasing serum 25(OH)D concentrations > 50–60 nmol/L and ensuring an adequate calcium intake is likely to significantly reduce fracture rates. There is also emerging evidence that this strategy will also be cost-effective, particularly in individuals aged > 50 years who are at increased risk of osteoporosis.
The role of calcium
The history of dietary recommendations on calcium
The first Australian recommended dietary intakes (RDIs) were issued by the National Health and Medical Research Council (NHMRC) in 1954. These RDIs have been subject to several revisions since, with the most recent revision being released in 2006.4
Before 1997 in the United States and 2006 in Australia, dietary recommendations were based on criteria where RDIs met the following definition:
Recommended Dietary Intakes (RDIs) are the levels of intake of essential nutrients considered … on the basis of available scientific knowledge to be adequate to meet the known nutritional needs of practically all healthy people. The RDIs are derived from estimates of requirements for each age/sex category and incorporate generous factors to accommodate variations in absorption and metabolism. They therefore apply to group needs. RDIs exceed the actual nutrient requirements of practically all healthy people and are not synonymous with requirements.93
Accordingly, earlier RDIs were not designed to evaluate the dietary adequacy of individuals, although they were often used or, indeed, misused for this purpose. In 1997, the US Institute of Medicine (IOM) developed a more complex framework for dietary recommendations that included the concept of adequate intakes (AIs), as well as estimated average requirements (EARs), recommended dietary allowances (RDAs) or intakes (RDIs, as used in Australia) and tolerable upper intake levels (ULs).94 These measures allowed for the evaluation of an individual’s dietary adequacy. Not all nutrients have both an EAR and AI; the AI is used when there is not enough evidence to set an EAR. It is important to note that intakes below the RDI cannot be assumed to be inadequate because the RDI by definition exceeds the actual requirements of all but 2%–3% of the population.
The 1997 revision of the dietary reference intakes (DRIs) for calcium, phosphorus, magnesium, vitamin D, and fluoride in the US set an AI for calcium.94 This revision was based on a different approach in response to expanded uses of the values and newer understandings of the role of nutrients. The concept of “optimal health” was introduced on account of the increasing acceptance that DRIs needed to extend beyond the prevention of deficiencies into the range of disease prevention. From 1997, a paradigm shift occurred with acknowledgement of the involvement of calcium in the aetiology of osteoporosis.95 Although reduced bone formation may aggravate the bone loss process in elderly people, particularly men, bone resorption is a major contributor to osteoporosis in women.96,97,98 As bone resorption is related to inadequate calcium intake, RDIs for calcium have risen steadily over the past 30 years. In the 2006 revision of nutrient reference values (NRVs), Nutrient reference values for Australia and New Zealand including recommended dietary intakes, the working party retained the concept of the avoidance of deficiency states as the concept used to set EARs and RDIs.4 Additional reference values were introduced to address chronic disease prevention. In setting the calcium requirements, the NRVs were based on calcium balance studies rather than changes in bone mineral density (BMD) or factorial estimates as used in the US DRIs, as this was considered to be problematic.4
In 2010, the US IOM released new recommendations for calcium,99 and set EARs and RDAs for calcium, rather than AIs.94 Box 5 provides a comparison of the revised recommendations from the US IOM with the current recommendations from the NHMRC. At the time of writing, the Australian Government has put to tender a scoping exercise to assess if a review is required of the current 2006 NRVs.
Food versus nutritional supplements for bone health
Food or food components may differ in their effects on bone compared with a single nutrient. Current thinking has moved towards examining associations with whole foods and food groups rather than single nutrients. This is based on the recognition that dietary components in food may interact.100,101 Additionally, most countries use a food-based approach to dietary guidelines, as this provides the best approach to inform dietary advice. However, a food-based approach presents issues when interpreting evidence relating to bone health:
- Many interventions have used a dietary calcium supplement, either with or without vitamin D.
- When a food-based intervention is undertaken, it is impossible to avoid changing the diet without altering the nutrient profile of the participant’s food intake.
- Milk studies are complicated by differences in composition (eg, in the US, vitamin D is routinely added to milk, whereas this is not the case in Australia).
- Few studies have been designed to address if food has a more favourable effect on bone compared with single nutrients.
There is a strong biological rationale for the importance of certain food groups in the maintenance of bone health. Grains, fruits and vegetables, meat and dairy, nuts and seeds supply a range of essential nutrients, including vitamins A, C, D and K, calcium, phosphorus, potassium, magnesium, and zinc, which have key roles in bone metabolism. There is considerable evidence of the positive influence that dietary patterns adequate in calcium, phosphorus and vitamin D have on bone health.48 Less consistent evidence exists on the role of other vitamins and micronutrients. Vitamin C is essential for production of collagen, the main protein in the bone matrix. Vitamin K is essential for the formation of the bone matrix protein, osteocalcin. While biochemical mechanisms associate these vitamins with bone mass, reduced intakes have been associated with low bone mass, increased bone loss and fracture,102 but the evidence for supplementation is limited or confusing.48
Potassium is considered to be important in achieving optimal bone health, due to its influence on calcium homoeostasis, particularly in the conservation and excretion of calcium, and may counter the negative effect of sodium on hypercalciuria.48 Only one supplementation study has shown the benefit of potassium citrate in older women consuming a high-salt diet compared with a placebo.103 However, many vegetables, fruits and dairy foods are good sources of potassium, and dietary patterns rich in these foods have been associated with reduced bone turnover in adults.104,105 Other minerals important for skeletal enzyme reactions include zinc and magnesium, which are present in legumes, vegetables and fruits. A large-scale US study has demonstrated that dietary patterns are related to BMD and, specifically, dietary patterns that include high intakes of vegetables and fruits result in significantly higher BMDs than those found with other dietary patterns.49 Therefore it seems prudent to encourage a varied diet, particularly including fruits and vegetables, to ensure adequate intakes of key nutrients to maintain bone health.
Dairy foods provide the major sources of calcium in the Australian diet, contributing 52% of the total calcium intake in men and 53% in women.106 In addition to its high calcium content, the other components of milk, including protein, lactose, magnesium and potassium, either alone or in combination with calcium, could also play an important role in bone growth and bone health. Intervention studies using milk in girls have reported positive effects on total body bone mineral accretion.107,108 A study of normally active boys with adequate calcium intakes found that additional exercise and calcium supplementation resulted in a 2%–3% greater increase in bone mineral content (BMC).109 A similar study in girls reported BMC increases of 2%–4% when short bouts of moderate exercise were combined with increased dietary calcium.110
Milk intervention studies in adults are limited, but have been evaluated in the following populations:
- Premenopausal women: Milk supplementation was effective in slowing bone loss.111
- Postmenopausal women: The rate of bone loss was slowed with milk supplementation in Chinese postmenopausal women with low calcium intakes.112 Another study directly compared calcium obtained from milk powder with calcium supplementation through tablets and reported equal effectiveness with both in slowing the rate of bone loss at the hip.113
- Middle aged and older men: A study of calcium- and vitamin D-fortified milk in older men found that the fortified milk stopped or slowed bone loss at the hip and spine and reduced cortical bone loss at the femur.114
The positive effects of milk consumption on fracture prevention have not been established.115
The effect of dietary protein on bone metabolism has long been debated. A recent systematic review and meta-analysis assessing dietary acid load and bone disease116 and several short-term controlled-feeding studies showed that a high-protein diet did not have adverse effects on calcium retention and bone metabolism.117,118,119 The positive effect of protein on bone health has been recently reviewed and the benefits to children and adults outlined.120 Notably, the provision of protein not only has an anabolic effect on bone, especially during periods of growth, but also improves calcium absorption.121 A recent systematic review showed that in older subjects, protein intake could explain 1%–2% of the variation in BMD,122 reduced bone loss over time,123,124,125 and reduced risk of hip fracture,126,127 although a recent placebo-controlled trial in older women did not show benefit to hip bone density with the addition of whey protein (30 g/day) to diet.128 However, it is important to note that benefits of protein on bone are greatest when calcium intake is in accordance with the recommended levels.129
Evidence of the benefits of a high fruit and vegetable intake in relation to bone density and osteoporotic fracture incidence is currently equivocal.105,130,131,132,133,134
Current calcium intake in Australia
The median dietary intake of calcium in the last Australian National Nutrition Survey was 827 mg per day for older men and 619 mg per day for older women (≥ 65 years).4 Thus, the median intake for men is close to the EAR of 840 mg per day, but for women the median intake needs to increase significantly in the oldest age group (EAR, 840–1100 mg per day).
In a random sample of Australian women with a similar median calcium intake of 631 mg per day, calcium intake from food sources alone was higher among those who also took a multivitamin supplement;135 only 7% of the women reported current use of calcium supplements. Inclusion of calcium derived from supplements increased the cohort’s mean total calcium intake by 6%. Calcium intake was not influenced by country of birth.
The 1995 National Nutrition Survey reported that 50%–66% of calcium intake was provided by milk products, with 30%–45% from dairy milk, about 10% from cheese and about 5% from frozen milk products.106 People who avoid dairy products need to ensure substitute food products are calcium-fortified. To achieve a daily intake of 1000–1300 mg calcium, at least three servings of dairy are recommended, with at least one of those servings being calcium-fortified. Studies suggest that the optimal level for calcium intake is higher when vitamin D status is low.8,44
A large randomised controlled trial (RCT) has demonstrated that long-term calcium intake in older women can be increased by providing an annual estimate of average daily calcium intake to the individual and her doctor.136 Strategies such as this feedback on dietary calcium intakes may become valuable tools in promoting increased calcium intake from food. Calcium and vitamin D supplementation have moderate to poor long-term adherence.16,137 These supplements are frequently perceived by patients as an excessive medication, and lack of motivation is the most common reason for non-adherence.138 Furthermore, calcium carbonate supplements are associated with gastrointestinal side effects such as bloating and constipation.139 Calcium citrate supplements have a lower proportion of elemental calcium, but the biological availability is higher; these supplements offer a good alternative with fewer gastrointestinal side effects, particularly in the elderly or those taking proton pump inhibitor drugs, which cause an elevation in gastric pH.
Benefits versus risks of calcium
Calcium plays an essential role in many physiological processes, including muscular, neural and metabolic functions, as well as bone mechanical properties. Studies suggest that a chronically negative calcium balance may contribute to suboptimal bone mass accrual in children, and to bone loss in adults.140,141,142,143 While potential side effects of calcium supplementation such as kidney stones, abdominal pain, hypercalcaemia and milk-alkali syndrome have been recognised for a long time, recent data on the cardiovascular safety of oral calcium supplements may challenge any recommendations, at least with regards to their use in the primary prevention of osteoporotic fractures.
Benefits of calcium
There have been numerous studies on the effect of calcium, with or without vitamin D supplementation, on bone turnover and BMD, both in healthy people and in patients with osteoporosis. Key findings include:
- Bone turnover and parathyroid hormone levels: Calcium supplementation alone appears to reduce parathyroid hormone (PTH) levels and bone turnover, particularly in people with low dietary calcium intake.144,145,146,147,148,149,150,151,152,153,154,155
- Bone mineral density (BMD): Inconsistent results have been observed in younger postmenopausal women in clinical studies.113,156,157,158,159 The same is true to an extent in studies of older people, although one larger study has described improved hip and whole body BMD.16 Additionally, a study of older Chinese women with low calcium intake reported reduced bone loss at the hip.160 A meta-analysis of smaller calcium-only trials concluded that calcium supplementation has a moderate but consistently positive effect on BMD in postmenopausal women.10 A recent meta-analysis found that supplementation with calcium, or calcium in combination with vitamin D, maintains or increases BMD at the spine and reduces bone loss at the hip.11 These effects seemed to be more pronounced in specific groups: in those with low baseline BMD or osteoporosis; in people with low dietary calcium intake or low vitamin D levels (< 25 nmol/L); in older subjects; and in women taking hormone therapy. It should be stated that there is a transient increase in BMD over the first 12 months of calcium supplementation followed by a reduced rate of bone loss compared with older women not taking calcium supplements.
The effect of calcium supplementation, with or without additional vitamin D, on fracture risk has been studied in diverse populations, including in healthy men and women living in the community, in patients with low bone density and osteoporosis, with or without prevalent fractures, and in the elderly. Key findings include:
- Primary fracture prevention: Two recent large-scale studies involving community-dwelling healthy postmenopausal women investigated the effect of calcium alone on osteoporotic fractures.16,161 Neither trial found a statistically significant effect of calcium supplementation on fracture incidence over 5 years. Poor compliance with medication may have contributed to these findings. A meta-analysis of studies of men and women living in the community proposed that calcium intake is not associated with hip fracture risk.12 However, this picture changes significantly in the elderly, particularly in those who are institutionalised, where supplementation with calcium and vitamin D is effective in reducing fracture risk, probably due to the fact that most elderly people are deficient in both.88,89,162 One study reported a reduction in the incidence of hip fracture by 43% during 18 months of treatment.88 A recent 19-year prospective cohort study of dietary calcium and fractures showed fractures were increased with dietary calcium intakes < 751 mg per day, but there was no further reduction with increasing dietary calcium intakes.8
- Secondary fracture prevention: Most, but not all, trials report no statistically significant effect of calcium supplements on fracture outcomes in patients who have already suffered osteoporotic fractures. The results of a meta-analysis10 were consistent with findings of the larger trials;13,163 that is, treatment of patients with osteoporotic fractures with calcium alone, vitamin D alone, or a combination of both without antiresorptive agents is insufficient to prevent further fragility fractures.
In summary, there is no good direct evidence that calcium, with or without vitamin D, prevents fractures in those who have already sustained a fragility fracture. However, the findings of most meta-analyses favour supplementation with calcium plus vitamin D to reduce fracture risk, although the overall effect may not be greater than a 10%–20% reduction in fragility fractures compared with placebo. Greater reductions in fracture risk (30% or more) have been observed in the elderly living in institutional care. Calcium intake significantly above the recommended level is unlikely to achieve additional benefit to bone health.
Potential risks of calcium supplementation
Calcium supplements can cause abdominal discomfort, constipation or, in some individuals, diarrhoea. These unwanted effects are usually dose-related, although not necessarily so.99 As a result of these relatively frequent side effects, long-term adherence to calcium supplementation is generally poor. The following serious complications have been subject to considerable analysis:
- Kidney stones: In susceptible patients, calcium supplements may cause or promote the formation of kidney stones. In postmenopausal women participating in the US Women’s Health Initiative study, supplementation with calcium (and vitamin D) was associated with a 17% increase in the risk of kidney stones.17 However, these findings may be attributable, in part, to the study protocol with a high baseline calcium intake, as a newer systematic review seems to indicate that there is no causal association between the risk of nephrolithiasis and calcium intake, be it via diet or calcium supplements.164
- Ischaemic heart disease: Recent reports from the University of Auckland have alerted the medical and scientific community to a potential association between calcium supplementation and an increased risk of ischaemic heart disease.18,165,166,167 Analysis of a 5-year study on the effect of calcium supplementation on fracture risk in postmenopausal women found the incidence of myocardial infarction (MI), as reported by the patient or her family, was significantly increased in women taking calcium supplements as compared with women taking placebo, but this difference was not significant when cases of MI were verified. A further meta-analysis also revealed a small but significant increased risk of MI or stroke.166 However, the risk of MI was not increased when the dose of the calcium supplement was < 805 mg/day. Findings from other studies are in contrast to the findings of the New Zealand group.168,169
Important issues in the New Zealand group studies are a lack of compliance with calcium supplementation (which in most trials was around 50%), randomisation of the additional strata used in the post-hoc analysis, adjudication of events, and questions regarding statistical data analysis. In the absence of an RCT of calcium supplementation with MI as a primary outcome (which is extremely unlikely), the question of whether calcium supplements cause cardiovascular complications or not will remain open for the foreseeable future. And so does the, perhaps, clinically most relevant question: “Does the potential risk of calcium supplements outweigh their proven but rather modest benefits?”
In order to reduce or prevent bone loss, it is vital to maintain an adequate intake of calcium. Overall, Osteoporosis Australia continues to recommend achieving a total daily calcium intake of 1000–1300 mg per day, depending on age and sex, and this should ideally be obtained from calcium-rich foods in the diet, by selecting foods high in calcium content, including calcium-fortified foods. However, when dietary intake of calcium is not sufficient, supplements may be required, at a daily dose of around 500–600 mg per day. Calcium supplementation, especially when it is combined with vitamin D, has been shown in clinical trials to reduce the rate of bone loss and has been an integral component in clinical trials with prescription medicines used to treat established osteoporosis.170
Nonetheless, special attention has recently been drawn to patients with significant renal impairment where calcium supplementation may indeed be associated with cardiovascular complications and, hence, negative clinical outcomes.171 In this patient population, caution may be warranted.
Effects of combination vitamin D and calcium on mortality
A recent meta-analysis of individual patient data on 70 528 randomised participants (86.8% female) with a median age of 70 years showed vitamin D, with or without calcium, reduced mortality by 7%.21 However, vitamin D alone did not affect mortality, but risk of death was reduced if vitamin D was given with calcium (hazard ratio, 0.91; 95% CI, 0.84–0.98). The number needed to treat with vitamin D plus calcium for 3 years to prevent one death was 151. This effect of the combination of calcium with vitamin D, which is not seen with either calcium or vitamin D alone, may be due to a greater effect of the combination in reducing PTH levels, which have been associated with both increased cardiovascular risk and increased mortality.
Calcium needs in children
Peak bone mass is acquired during childhood and sets the stage for vulnerability to fracture and other bone disorders. Severe calcium deficiency during infancy can exacerbate vitamin D deficiency and lead to rickets.172 Calcium deficiency rickets typically occurs after weaning and often after the second year of life.173 Healing of rickets in Nigeria and South Africa has occurred from calcium supplementation without vitamin D.174
The next most vulnerable period for inadequate calcium is during puberty, when about 40% of adult peak bone mass is acquired.78 Various skeletal sites reach their peak at different rates. In girls:
- peak total body BMC is acquired by age 22 years175
- at the hip, greater trochanter reaches peak bone mass at 14.2 years and the femoral neck at 18.5 years176
- the spine reaches peak bone mass by age 23 years.176
Early puberty is a period of high fracture prevalence, partly due to low BMD as peak height velocity occurs before peak BMC accrual.177 Vulnerability to fracture is also associated with low milk consumption.178 Calcium intake is primarily related to consumption of milk and milk products, as supplement use is low in children.179 Fracture incidence in children has increased in recent decades; this has been attributed to decreased milk consumption, decreased physical activity and increased body fat.180 When an obese child falls, greater force is exerted on the outstretched radius. In this situation, increased body weight is transmitted through the limb, resulting in a force that may exceed the strength of the bone and so lead to fracture.181 Increasing body mass index (BMI) is associated with increased calcium needs.182 Thus, in the prevalent situation of increasing BMI with inadequate calcium intakes, overweight children have proportionally low bone mass.183 Increased calcium intakes in obese children may result in stronger bones that would resist fracture.
Calcium recommendations during childhood should be aimed at optimising skeletal accrual to produce the highest peak bone mass within a child’s genetic potential. During infancy, calcium provided by breast milk is assumed to be adequate and is typically the basis for recommended intakes (see Box 5). Almost all of the experimental evidence for setting calcium requirements during childhood is related to adolescents. Two main approaches to determining calcium required have been used: the factorial approach99,184 and intakes for maximal calcium retention.94 The recommendations using both approaches for adolescents are consistent.
A meta-analysis of RCTs of calcium supplementation in children6 suggests that increasing calcium intake from a mean 700 mg per day to 1200 mg per day has only limited benefits for improving bone acquisition. There was no effect on BMD at the femoral neck or lumbar spine, and only a small effect on total body BMD, which did not persist when supplementation ceased, suggesting a lack of long-term benefit. There was a small persistent effect on upper limb bone mass that was unlikely to be of clinical importance in terms of fracture prevention. In addition, there was no evidence to suggest that increasing the duration of supplementation led to increasing effects, or that the effect size varied with baseline calcium intakes, down to levels < 600 mg per day. In a subsequent 18-month trial in children (mean age, 12 years) with a habitual calcium intake < 650 mg per day, supplementation resulted in greater increases in bone mass at all sites but, again, these effects did not persist once supplements ceased.185 Thus, evidence does not support the use of calcium supplements in healthy children, with the possible exception of those with very low calcium intakes. This may not apply to children with medical conditions affecting bone metabolism. It is unclear whether this reflects calcium deficiency and/or protein deficiency but, in the absence of strong evidence, children who avoid dairy should be encouraged to improve their calcium intake from other dietary sources, and supplementation should be considered if necessary.
Regardless of the requirements for calcium determined for children around the world, calcium intakes are often inadequate. Analysis of calcium intakes for 20 countries shows that among adolescent children, the intake for boys was about 60% and for girls about 50% of a particular country’s specified requirement.186 Achieving optimal nutrition including calcium and physical activity during growth is an important investment for society. The health care costs and losses in quality of life related to osteoporosis later in life are growing. Strategies to build peak bone mass during growth have the greatest potential for return on investment.
Calcium needs in healthy adults
Although the key role of adequate calcium nutrition in the prevention of osteoporotic fracture is well established, there is little evidence relating directly to the role of calcium intake in maintaining bone mass or fracture prevention in young and middle-aged adults. RCTs investigating this age group require large sample sizes to demonstrate fracture reduction. Fracture rates are low in those aged under 50 years, and a high proportion of those that occur are associated with high-trauma events, making it difficult to identify bone fragility.187 Analysis of RCTs has also been hampered by the high baseline calcium intakes of middle-aged and older men.188 Significant change to outcomes related to bone mass is difficult to demonstrate, as bone mass is relatively stable in young adulthood.189 Our current knowledge relating to adults aged under 50 years has largely been derived from studies using specialist groups such as elite athletes and religious groups with strict dietary restrictions.190,191,192
RCTs assessing bone density or mass generally show increases following calcium intake and/or supplementation compared with placebo, typically between 1% and 2% (absolute difference over 2–3 years). Similar beneficial effects on bone health in perimenopausal women have been reported in some,193 but not all, studies.10 Although calcium is a key nutrient in bone health, it is difficult to adequately power randomised trials, as the benefits of increased calcium intake on bone parameters are modest and there is substantial individual variation in rates of bone loss among perimenopausal women.
A recent meta-analysis of RCTs suggests an increased risk of cardiovascular events in those using calcium supplements.18 The current controversy relating to the risk–benefit ratio of calcium supplementation has translated to a less consistent message from experts regarding calcium supplementation. Nevertheless, this controversy is restricted to the use of supplements, and the recommendations for an adequate dietary calcium intake are consistent and remain unchanged from the current position paper.7 An adequate calcium intake achieved through diet continues to be the best choice for those who can include an adequate dairy product intake. Strategies to increase dietary calcium intake and sustain an adequate intake over the long term136 will become more important as the debate on the risk–benefit balance of calcium supplementation continues.18,19,137
The Australian and New Zealand RDIs for calcium are 1000 mg per day in women aged 19–50 years and men aged 19–70 years. This increases to 1300 mg per day for women aged over 50 and men aged over 70 years. This allowance is calculated to meet the needs of 95% of the population.4 The corresponding EARs are 840 mg per day and 1100 mg per day for the younger and older age groups, respectively.4 In practice, this translates to 3–4 serves of calcium-containing foods or 2–3 servings of high-calcium foods each day.
There is some evidence that calcium supplementation or fortification in men and young women before menopause is beneficial, but most research has focused on postmenopausal women. There is no evidence to suggest that individuals consuming calcium at levels significantly higher than the requirement are receiving additional benefit.7,8 Thus, a well designed study investigating the effect of calcium fortification in food, with and without exercise, has not demonstrated any additional benefits of calcium-fortified milk on bone parameters, as the baseline calcium intake of the male participants was already at the recommended level.188
Calcium needs in older adults and individuals with osteopenia and osteoporosis
Physiologically, calcium phosphate or hydroxyapatite provides rigidity to the skeleton, but bone calcium also acts as a reserve to maintain the concentration of ionic calcium in the extracellular fluid within a crucial narrow range. The PTH–vitamin D system maintains plasma calcium at the expense of the skeleton. Calcium deficiency reduces bone mass by increasing bone resorption to preserve this extracellular fluid ionised calcium level. Vitamin D deficiency may cause osteoporosis by secondary hyperparathyroidism and increased bone resorption. Thus, individuals who develop osteopenia or osteoporosis may have a low calcium intake and/or reduced calcium absorption related to vitamin D deficiency.
Meta-analyses and RCTs with a primary outcome of fracture provide the highest level of evidence supporting the crucial role of calcium nutrition in the prevention of osteoporosis and fragility fractures.9,10,11 However, there is significant heterogeneity in the results,12,13,14,15 with differences in dose, baseline nutrient status, and co-administration of vitamin D, as well as poor adherence, all contributing to the inconsistency in results. One meta-analysis concluded that supplementation with calcium plus vitamin D reduced the relative risk of fractures by 12% in adults aged 50 years and older.11 For best therapeutic effect, doses of 1200 mg calcium and 800 IU (20 µg) of vitamin D were recommended. It is generally regarded that calcium supplementation prevents fractures in the frail elderly, particularly in women in residential care.11 The frail elderly have the highest rates of fracture, and those in residential care typically have low vitamin D status and an inadequate intake of calcium. Thus, this group of individuals has the greatest potential to benefit from either an increased intake of calcium alone or calcium plus vitamin D. Nevertheless, calcium intakes well above the recommended allowance of 1300 mg per day are not associated with any additional benefit, and calcium supplementation may be associated with an increased risk of hip fracture.8,12,15,194
Supplemental calcium, either alone or combined with vitamin D, is associated with a reduced rate of bone loss averaging 0.5% at the hip and 1.2% at the spine.11 Consequently, calcium supplementation is generally regarded as having only a modest suppressive effect on bone remodelling.195 Also, the apparent gain in BMD in the first 6–12 months after commencing calcium supplementation overstates any sustained benefit. In the immediate period, the increased calcium intake suppresses bone remodelling, resulting in a transient perturbation of the steady state of bone turnover. Furthermore, there is little evidence to support any effect of calcium supplementation after 4 years,196 although in older men, there is some evidence that the skeletal benefits gained from consuming calcium–vitamin D-fortified milk over 2 years are sustained up to 18 months after withdrawal of the milk.197
Several well designed randomised trials on calcium supplementation have reported no significant effect using intention-to-treat analysis.11,13,15,16 Poor adherence rates (55%–60%) to study medication have commonly been attributed as contributing to these results, as some “per protocol” analyses have demonstrated benefit.14,17 The most common side effects of calcium supplements relate to bloating, constipation, difficulty in swallowing a large tablet, and, less commonly, a slightly increased risk of kidney stones. While poor adherence to calcium supplements has been a public health concern, the emphasis has changed to a re-evaluation of the risk–benefit ratio of calcium supplementation. Evidence from recent re-analyses of some trials has suggested the use of these supplements may be associated with an increased risk of cardiovascular events.18 The evidence is mounting, although the debate heightens, as few of the trials re-analysed were designed to investigate cardiovascular outcomes and the ascertainment of these events could be biased.19 The most recent re-analysis of the Women’s Health Initiative dataset and meta-analysis concludes that calcium supplements with or without vitamin D modestly increase the risk of cardiovascular events, especially MI. The authors call for a reassessment of the role of calcium supplements in the management of osteoporosis since the beneficial effect on fractures is also small.18,137 Other investigators take a different view,198 highlighting the need for ongoing evaluation and discourse on this subject in the scientific literature.
Current evidence does not demonstrate a cardiovascular risk with dietary calcium intake or that the associated benefits of increased high-quality protein in the elderly, who increase their intake of dairy foods, will translate to benefit to overall health status.
The role of vitamin D
Current issues
There is reasonable agreement, based on a considerable body of evidence, that the vitamin D system is a critical contributor to calcium and phosphate homoeostasis and is important for optimal bone and muscle function. On many other matters, there is considerable controversy and uncertainty. Sun exposure is the primary source of vitamin D, in accordance with current Australian and New Zealand Bone and Mineral Society (ANZBMS), Endocrine Society of Australia (ESA) and Osteoporosis Australia (OA) recommendations.42
What constitutes an optimal level of vitamin D?
What should be the target for vitamin D sufficiency? Based on a large amount of evidence, a conservative target level for 25-hydroxyvitamin D (25[OH]D) for adequate calcium homoeostasis and reasonable bone and muscle function has been suggested as at least 50 nmol/L.42,99,199 The best time to measure serum 25(OH)D is at the end of winter/early spring when levels are at a nadir. It is recognised that this is conservative and, on the basis of an autopsy study of bone histology and other data, a target of > 75 nmol/L may be more appropriate.200 At the very least, if the 25(OH)D level is measured around the end of summer, allowance needs to be made for a subsequent drop during winter, so that a higher target of at least 60 nmol/L may be advisable.42 Box 3 represents the current scientific findings from the vitamin D position statement supported by the ANZBMS, the ESA, and OA.42
Since vitamin D receptors are present in all nucleated cells examined and many cells have the capacity to produce the active hormone, 1,25-dihydroxyvitamin D (1,25[OH]2D), there is considerable interest in the possible extraskeletal effects of this hormone. There are extensive laboratory experimental data over many years supporting these proposed effects — one of the first demonstrations that 1,25(OH)2D was important for insulin secretion was published in 1980.201 There is also a relatively large body of supporting data from studies of autoimmune diseases, cancer studies and models of innate immunity in animals. The human studies, however, are mostly limited to epidemiological studies showing, with moderate consistency, that high sunlight exposure or other indices of replete vitamin D status are associated with reduced risk of certain cancers and autoimmune diseases, such as type 1 diabetes and multiple sclerosis, as well as being involved in a range of other health parameters. Although such observations have been made since 1937,202 evidence supporting a role for vitamin D in these non-skeletal health outcomes from well conducted randomised controlled trials (RCTs) is mostly lacking. The trials that have been conducted have mostly not had extraskeletal health effects as a primary outcome, while the trials that have had extraskeletal health effects as a primary outcome have tended to be small, short and have had dose or compliance problems. Large-scale trials, such as the Vitamin D and Omega-3 Trial (VITAL), are underway, but will take some time to report. There are also ethical issues in undertaking the trials. Ideally, the enrolled subjects should be vitamin D deficient/insufficient (by defined criteria), as increasing vitamin D levels beyond some optimal concentration may produce no further benefit; however, this poses a problem for the placebo group.
Some indicators have started to appear in the literature that high 25(OH)D concentrations, mostly > 100 nmol/L but sometimes > 75 nmol/L, may be associated with adverse health outcomes.203,204,205 The data are surprising, considering that the 25(OH)D levels of people living in high ambient ultraviolet (UV) environments average around 130 nmol/L.206 The nature of the studies showing these negative effects is similar to that of the epidemiological association studies showing better health outcomes from higher 25(OH)D levels.
Some of the discrepancies in the literature may be due to genetic influences on vitamin D status and response to therapy. Polymorphisms of the genes encoding vitamin D binding protein, 7-dehydrocholesterol reductase, which affects substrate levels in skin, and the putative 25-hydroxylase have been shown to affect vitamin D status.207 More recently, the effect of vitamin D on tuberculosis seroconversion has been reported to be dependent on polymorphisms of the vitamin D receptor,208 while infantile hypercalcaemia in response to moderate supplemental vitamin D has been shown to be a consequence of mutations in the 24-hydroxylase gene.209
Vitamin D storage and metabolism
There is little understanding of vitamin D storage. The secosteroid, 25(OH)D, has a half-life in blood of 15–50 days, much greater than most steroids and much greater than that of its binding protein.210,211 Little is known about the factors which affect half-life, except that low calcium intake and/or high parathyroid hormone (PTH) levels markedly shorten this.210,212 Vitamin D can be given as a yearly dose, with reasonable maintenance of 25(OH)D levels over most of the year.213,214 Some vitamin D goes into fat, where it appears to be trapped.215 Some 25(OH)D goes into muscle,216 but meat is a poor source of vitamin D.
Many of the physiological effects of vitamin D, though not all, are better related to circulating 25(OH)D levels than to 1,25(OH)2D concentrations. The latter are poor indicators of vitamin D status.217 This may be explained in part by the ability of many tissues, including bone, macrophages and probably parathyroid gland, to convert 25(OH)D to 1,25(OH)2D locally. Indeed, in laboratory studies of bone cell function, endogenously produced 1,25(OH)2D caused different functional effects from exogenously added hormone.218
Protocols for vitamin D administration
Assuming there is a need to improve a patient’s vitamin D status and that advice to increase sun exposure is impractical or inadvisable, what is an appropriate protocol? Most vitamin D supplements in Australia are vitamin D3. This means the controversies about whether vitamin D2 raises 25(OH)D levels as effectively as vitamin D3 (even if the assay measures both adequately) and, more importantly, whether vitamin D2 is less effective functionally (on which there are very few recent data) are not major issues in this country.219,220 There is insufficient appreciation of data that show that standard 1000 IU (25 µg) doses of vitamin D per day can be expected to raise 25(OH)D levels by only 10–20 nmol/L.221,222 Major issues are cost and compliance, particularly in refugee communities and the elderly. For these reasons, weekly, monthly, 3-monthly or yearly doses of vitamin D have been advocated. Generally available vitamin D supplements are usually 1000 IU (25 µg) oral tablets or capsules, liquid vitamin D (1000 IU/0.2 mL), and preparations imported with permission or made by compounding chemists. Intermittent, high-dose vitamin D (eg, 50 000 IU per month) is cheaper, effective in improving vitamin D status quickly,223 produces average 25(OH)D levels consistent with the equivalent daily dose224 and does not seem to cause undue problems with hypercalciuria or hypercalcaemia.213,223 However, while dosing of 100 000 IU (2500 µg) every 4 months was shown to reduce fractures in a community study in the UK,86 a 500 000 IU (12 500 µg) yearly dose of vitamin D3 in winter in Victoria resulted in increased falls and a tendency to increased fracture rates in the first 3 months following the dose.214
Even if optimal-dosing protocols for vitamin D could be established, most meta-analyses of RCTs examining falls and fractures (and a recent one on overall mortality) report that improved outcomes are generally the result of combined treatment with vitamin D and calcium, rather than either agent alone.11,225,226 Calcium supplements are normally given daily and are often combined with vitamin D.
Vitamin D deficiency in Australia
The definition of vitamin D sufficiency has varied among the studies conducted in Australia. Regardless, as a population, it is evident that Australians are not as vitamin D sufficient as might be expected for residents of a “sunny country”. A study combining results from mostly normal populations in south-east Queensland, Victoria and Tasmania reported a prevalence of vitamin D deficiency (defined as < 50 nmol/L) in women in winter and spring of 40% in Queensland, 37% in Victoria and 67% in Tasmania.24 Seasonal differences were clear in all groups, with vitamin D values falling along with the peak UV index. Similar findings were reported from a study in south-east Queensland of men and women in the age range 18–87 years;32 at the end of winter, the prevalence of vitamin D deficiency was 42%. A study of nearly 200 blood donors of both sexes in winter and spring in Perth reported that 34% had 25(OH)D levels < 50 nmol/L.227 The largest study of nearly 11 300 Australians from Darwin to Hobart showed that 31% had 25(OH)D levels < 50 nmol/L.23 Limited evidence suggests that vitamin D levels are lower in those living in urban areas compared with country dwellers and lower in women compared with men.228 Reasonably consistent data suggest that vitamin D levels increase with physical activity and decrease with obesity.215,229,230,231,232
Vitamin D insufficiency has been reported in 10% of 8-year-old Tasmanian children in winter and spring233 and in 68% of 16–18-year-old boys in winter.234 These results may be a consequence of less time spent outdoors in the teen years, as longer time spent outdoors in winter, vigorous activity and involvement in more sports all correlated with higher 25(OH)D levels in teens.234 In pregnant women, 25(OH)D levels seem similar to those reported for the rest of the adult population. In country Victoria in winter, 35% of pregnant women had 25(OH)D values below 50 nmol/L; the corresponding rate in summer was 15%.235
Older individuals, particularly those living in aged care facilities, are at high risk of vitamin D deficiency.26,27,28 The skin of older individuals is thinner,236 which may explain lower concentrations of 7-dehydrocholesterol substrate (pre-vitamin D) in skin of older individuals,237 and, in turn, the observation that older people make less vitamin D under conditions of high UV exposure.238 However, older people exposed to smaller amounts of UVB appear to synthesise similar amounts of vitamin D compared with younger people.39,239 A much greater problem is limited sun exposure, due to frailty, reduced mobility or preference.240
Other groups at greatly increased risk of vitamin D deficiency include:
- individuals with dark skin29,30,31
- individuals who wear modest dress29,30,32
- groups at high risk of skin cancer due to past history or immunosuppression33,34,35
- individuals with malabsorption36
- individuals less likely to spend time in the sun, including chronic disease sufferers, transplant recipients, and office and shift workers35
- individuals taking antiepileptic medications37,38
Somewhat surprisingly, sunscreen use in the general population is not associated with low vitamin D levels, despite the capacity of sunscreen to block most UVB in the laboratory.32,35,39 Inadequate application combined with generally higher sun exposure in individuals using sunblock probably explains the discrepancy.35,39
Low vitamin D levels will be found in people with no obvious risk factors. Whether this can be entirely explained by genetic variations in genes encoding such key proteins as 7-dehydroreductase, vitamin D binding protein or 25-hydroxylase,207 or by factors still unknown that affect metabolism or storage, remains to be studied.
Vitamin D testing
The use of vitamin D testing has grown exponentially in recent times as the result of increasing interest in the role of vitamin D in health.241 Within the body, vitamin D (either ergocalciferol or cholecalciferol) is hydroxylated in the liver to the major circulating metabolite 25(OH)D, which is in turn converted in the kidney to the active hormone 1,25(OH)2D. Despite being the precursor metabolite, total circulating 25(OH)D (combined 25[OH]D2 and 25[OH]D3) is accepted as the best measure of vitamin D status.242 Compared with 1,25(OH)2D, 25(OH)D concentrations in serum are higher and less tightly regulated, as well as being more stable.243 This renders 25(OH)D a good indicator of vitamin D stores and makes the quantification of vitamin D quite unusual, being one of the few clinical situations in which the metabolite one step removed from the active hormone is used to assess adequacy.
Several assays for 25(OH)D measurement are in common use. Radioimmunoassay methods have been superseded by automated immunoassays using chemiluminescence technologies, such as DiaSorin Liaison Total and IDS iSYS. These assays are easily set up and are capable of high-volume throughput, lending their use to many clinical laboratories in Australia.
Non-immunological direct detection assays, such as high-performance liquid chromatography (HPLC) and liquid chromatography–tandem mass spectrometry (LC-MS/MS), have added advantages in performance, including the ability to independently measure 25(OH)D2 and 25(OH)D3 and discriminate 3-epi 25(OH)D3, superior low-limit quantification, and the ability to control for standardisation. However, without adequate controls, reliability of results is highly operator dependent.244 Previously limited to specialist laboratories, procedures to automate these methods and to increase throughput will enable HPLC and LC-MS/MS to be increasingly used in clinical laboratories.245
At present, LC-MS/MS is considered by many commentators as the gold standard methodology.246 This assay is the preferred method for measurement of 25(OH)D concentrations in the UK National Diet and Nutrition Survey,244 the US National Health and Nutrition Examination Survey (NHANES)245 and the Australian Health Survey.247 Despite this, concerns regarding assay reliability persist and have been well documented in the literature.248,249,250,251,252,253 An international vitamin D standardisation program is being led by the US Office of Dietary Supplements.254 The Australian Health Survey is participating in this program, along with a number of national population health surveys from other countries.
This uncertainty has substantial implications for clinicians when interpreting vitamin D results. A single 25(OH)D measurement may incorrectly classify a patient as vitamin D deficient and result in unnecessary treatment with vitamin D supplementation. Alternatively, a patient being monitored for vitamin D deficiency could have a change in apparent clinical status merely from variability in measurements at the same laboratory or from having serum analysed using a different assay method or laboratory. Clinicians need to recognise the limitations of current assays and seek guidance from their laboratories as necessary; for example, regarding normal reference intervals. The uncertainty around assay reliability also affects the comparison of the prevalence of low vitamin D levels in surveys using different laboratory methods.
There is a need for standardisation of 25(OH)D measurement methods to enable calibration of assays, similar to the DEQAS (Vitamin D External Quality Assessment Scheme) operating in the UK. This has led to the US National Institute for Standards and Technology developing a standard reference material (SRM) to aid in vitamin D analysis. SRM 972 Vitamin D in Human Serum consists of four pools of human serum with known analyte values for vitamin D metabolites, including 25(OH)D2, 25(OH)D3 and 3-epi 25(OH)D3.255 Adoption of SRM 972 represents the way forward as the first step in calibrating assays and serving as an adjunct to quality assurance programs. SRM 972 use has been broadly accepted in major health surveys244 and will enable greater confidence in the reliability and reproducibility of 25(OH)D testing. Ultimately, this will enable clinicians to implement best practice in the treatment of vitamin D deficiency.
Vitamin D from sunshine versus vitamin D from supplements
Overexposure to sunlight, specifically ultraviolet radiation (UVR), is associated with adverse events, the most serious of which is skin cancer, including melanoma. As a consequence, there is much expert conjecture about the concept of deliberate UVR exposure as a means of attaining “optimal” vitamin D status.256 Current recommendations of intentional UVR exposure suggest that a 10–15 minute exposure of the face, hands and arms (about 25% of the body surface area) 2–3 times a week in the spring, summer and autumn corresponds to an equivalent oral dose of 1000 IU (25 µg) vitamin D. This is said to be adequate to satisfy the body’s requirement for vitamin D throughout the year.257,258
This regimen has led to a significant increase in serum 25(OH)D levels in select populations.259,260,261 However, modelling of short regular UVR exposures has shown them to be of little benefit in maintaining vitamin D adequacy in the general population and could compromise skin health.262
Environmental, behavioural and genetic factors have been shown to influence the photosynthesis and bioavailability of vitamin D, and make it difficult to carefully and universally “titrate” an individual’s sun exposure.263 Known modifiers of vitamin D status include:
- sun protection practices264
- ambient UVR265
- latitude266
- skin colour29,267
- genetic predisposition263,268
- sex238,269
- age, weight and height270,271,272
- socioeconomic status182
- the incidence of several chronic illnesses.273
Vitamin D occurs naturally in a limited number of foods, with oily fish being the richest natural source of the vitamin. In Australia, margarine and some milks and milk products are currently fortified with vitamin D,274 and vitamin D-fortified mushrooms and bread baked with high vitamin D yeast are shortly coming into circulation.275,276 However, dietary food sources of vitamin D tend to be sporadic and varying in concentrations, and some controversy exists over whether or not vitamin D2 can fully substitute for vitamin D3 in the human diet.220,277
The average dietary intake of vitamin D is estimated to be 104–120 IU (2.6–3.0 µg) per day for men and 80–88 IU (2.0–2.2 µg) per day for women, while the adequate intake (AI) of vitamin D is 600 IU (10 µg) per day and higher for older people.274 These AIs assume there is minimal sunlight exposure. However, adequate intake of vitamin D is unlikely to be achieved through dietary means, and the main source of vitamin D in healthy adults is the vitamin D produced in the skin.
Vitamin D supplements provide a safe, accessible and relatively inexpensive means of increasing vitamin D intake, although opinion is divided on the recommended daily dose.40,278,279 The International Osteoporosis Foundation recommends taking a vitamin D supplement of 800–1000 IU (20–25 µg) per day to achieve adequate blood levels of 25(OH)D, defined as 75 nmol/L.47 Some experts support a higher “optimal” serum level of 25(OH)D of 75–110 nmol/L and suggest that a daily supplement of 4000 IU (100 µg) per day is required.92,280 People at high risk of deficiency, including those who are severely overweight and spend little time in the sun, may require even more supplementation.281,282 Although current knowledge about the effects of taking such high doses of vitamin D for sustained periods is limited,17,283,284 a recent review has suggested that as much as 10 000 IU (250 µg) per day is a safe upper intake for adults.285 To date, high-dose supplementation has not been shown to provide greater benefit than more moderate doses.286
It is clear that many people require more vitamin D than they are currently able to produce through sun exposure to achieve a serum 25(OH)D level > 50 nmol/L. Current understanding of the complex interaction of genetic, behavioural and environmental factors that influence the production of vitamin D within human skin is limited. While UVR exposure is an effective means of increasing vitamin D status, deliberate UVR exposure for durations sufficient to increase vitamin D status may increase the risk of other adverse health outcomes. Accordingly, daily oral supplementation remains the most safe, reliable and effective method to increase vitamin D levels.
Vitamin D fortification in food
Few foods contain significant amounts of vitamin D. Small amounts of vitamin D3 are found in the fat of animals and, as such, full-cream milk and butter contains vitamin D, but the amount is dependent on the season of production. A rich source is fish, especially high-fat fish such as salmon, herring and mackerel from the North Sea. It should be noted that farmed salmon contains only one-quarter of the amount of vitamin D found in wild salmon, and vitamin D can be lost in the cooking process. For many countries, foods fortified with vitamin D are the major dietary sources of vitamin D. In Australia, all margarines are mandatorily fortified with small amounts of vitamin D. Small amounts of vitamin D are permitted to be added to dried milk,287 modified milk, cheese, yoghurt, dairy desserts, butter, various analogues derived from legumes and their products, certain beverages derived from cereals, and formulated beverages, but few milks are fortified in Australia. UV-irradiated mushrooms also contain vitamin D. It is not permitted to add vitamin D to breakfast cereals or fruit juices in Australia, in contrast with many other developed countries.
The intake of vitamin D in Australia is less than the intakes recorded in countries that either mandate vitamin D fortification of milk at levels higher than those allowed in Australia and/or permit extensive voluntary vitamin D fortification of a number of food products. Under these circumstances, the mean vitamin D intake for adults can be almost double the Australian intake, at about 192 IU (4.8 µg) per day.288
The only country to employ mandatory vitamin D fortification of milk at a level twice that allowed by Australia is Canada (~ 80 IU [2 µg] per 200 mL). The mean vitamin D intake from food in Canada is 232 IU (5.8 µg) per day in adults, with higher intakes seen in children; median intakes of 1–3-year-olds and 4–8-year-olds are 252 IU (6.3 µg) per day and 224 IU (5.6 µg) per day, respectively.288 Vitamin D fortification at this level appears to have some positive impact on the rates of severe deficiency and rickets. A recent analysis of a representative sample of Canadians indicated that, in winter, 25% were classified as deficient (< 50 nmol/L), and that overall only 5.4% had levels in the moderate deficiency range (< 30 nmol/L),99 which rose slightly to 7% in winter. These rates of deficiency in the general population are somewhat lower than those reported in the Australian Diabetes, Obesity and Lifestyle (AusDiab) study.289 In AusDiab, overall 31% of men and women were deficient (< 50 nmol/L) and 4% were moderately deficient (< 25 nmol/L), increasing to 8.9% of women being moderately deficient during winter (June – August).290
The fortification of food products is becoming frequently used to improve calcium intake and this may also be a reasonable method to increase the vitamin D intake of the population.102 It is clear that the current food supply, supplementation practices, and lifestyles in most developed countries result in large segments of their populations being at risk of vitamin D deficiency. This is particularly relevant to Australia, which has a diverse immigrant population who cannot achieve sufficient safe sunlight exposure to maintain adequate levels of serum 25(OH)D; immigrants from North Africa, the Middle East and Asia in particular are at high risk of vitamin D deficiency. Almost three-quarters of pregnant women from the Horn of Africa living in Melbourne had serum 25(OH)D levels < 25 nmol/L.31
It is possible that a modest level of mandatory vitamin D fortification in milk, as is currently employed in Canada, in combination with voluntary permission to fortify other food products, such as breakfast cereals and fruit juices, may be effective in reducing the level of severe deficiency and also the incidence of rickets in children. Levels of vitamin D food fortification in Australia are currently insufficient to prevent deficiencies. Expanding the fortification of the food supply is likely to be effective in correcting severe deficiencies in high-risk groups. However, more evidence on whether there is widespread vitamin D deficiency is required before such a case can supported.
Vitamin D needs in pregnancy and in children
Vitamin D needs in pregnancy
During pregnancy, alterations to vitamin D and calcium homoeostasis allow calcium transfer to the developing fetus. Levels of 1,25(OH)2D and vitamin D binding protein increase throughout pregnancy, absorption of intestinal calcium is doubled and PTH is suppressed to the lower end of the normal range in situations where vitamin D and calcium intake are adequate.291 The fetus is dependent on maternal vitamin D and there is a strong association between maternal and cord blood vitamin D levels, although cord blood levels are about 65% of maternal levels.292
Evidence is limited for the effect of vitamin D on maternal bone health during pregnancy, although there is emerging evidence that vitamin D may be protective for a range of other pregnancy outcomes, including pre-eclampsia,293,294,295 gestational diabetes,40,296,297,298 bacterial vaginosis299 and pregnancy complications.300,301
Longitudinal studies of bone mineral density (BMD) during pregnancy provide conflicting results, although most studies show decreases in BMD at the spine, hip and distal radius.302 There are no studies directly examining the relationship between vitamin D status and BMD during pregnancy.302
Both observational studies303,304,305,306 and RCTs301,307,308,309,310,311,312 in pregnant women have found vitamin D supplementation increases maternal circulating 25(OH)D levels, but most have found no effect of increased vitamin D on maternal calcium301,303,304,306,309,310 or PTH levels.305,309 Two RCTs of vitamin D supplementation in vitamin D-deficient women have found improvement in maternal calcium levels (with an increase in mean 25[OH]D levels from 20 nmol/L to 168 nmol/L)307 and a reduced prevalence of maternal hyperparathyroidism (with an increase in median 25[OH]D levels from 26 nmol/L to 42 nmol/L).311
There is conflicting evidence on the influence of maternal vitamin D status on fetal growth. Two prospective cohort studies have evaluated maternal 25(OH)D and birth weight: one found no association,313 while the other found a non-significant association between low maternal vitamin D and reduced knee–heel length, but no association with other infant growth parameters.314 This group later reported the relationship between birth weight and maternal vitamin D was modified by vitamin D receptor genotype.315 A retrospective cohort study found no relationship between maternal vitamin D and infant birth weight or length, although it did find a relationship between maternal vitamin D status and subsequent BMD in the children.316 Two further observational studies (vitamin D levels not measured) have found higher birth weight in babies born to women with higher intakes of dietary/supplemental vitamin D during pregnancy.317,318
Three RCTs of vitamin D supplements in vitamin D-deficient women (two from the same group, all with methodological issues) have found fewer infants who are small for their gestational age,308,312,319 a smaller fontanelle size,319 and improved postnatal infant growth308 in infants born to women in the intervention group. A further poor-quality RCT of high-dose vitamin D in Indian women during pregnancy (two doses of 600 000 IU [15 000 µg] during third trimester) found increased birth weight and length, although maternal vitamin D was not measured.320 Other trials of vitamin D supplementation in pregnant women with low vitamin D310,311,321 have not found any difference in birth weight.
Javaid and colleagues316 found maternal vitamin D ≤ 27.5 nmol/L in late pregnancy was associated with reduced whole-body bone mineral content (BMC), bone area and areal BMD in children at 9 years compared with children where maternal vitamin D was > 50 nmol/L, suggesting low vitamin D during pregnancy is associated with persisting deficits in bone mineral accrual.316 Recent prospective cohort studies also suggest maternal vitamin D status influences fetal bone parameters. Maternal vitamin D < 50 nmol/L is associated with increased femoral splaying in the fetus measured by high-resolution 3D ultrasound,322 and maternal vitamin D < 42 nmol/L is associated with lower tibial BMC and reduced cross-sectional area in neonates measured by peripheral quantitative computed tomography (pQCT),323 with a reduced tibial size persisting at 14 months of age.324 Conversely, a study of Gambian infants found no relationship between maternal vitamin D status during pregnancy and BMC, bone width, bone area or BMD during the first year of life, although 80% of mothers had vitamin D > 80 nmol/L.325 A study of Asian neonates in the UK found no difference in the BMC of infants born to mothers receiving vitamin D supplements compared with unsupplemented women, although in this study, mean cord blood 25(OH)D was < 15 nmol/L in both groups.321
There have been no studies addressing whether optimal 25(OH)D levels in pregnancy are different from optimal levels in non-pregnant women.291 The current recommended adequate vitamin D levels for adults are > 50 or 60 nmol/L, although it is noted that some authors recommend a higher target level of around 80 nmol/L during pregnancy,300,326 and there is emerging randomised trial evidence of improved pregnancy outcomes with vitamin D levels > 100 nmol/L.301
Supplementation trials in pregnant women with low vitamin D (mean/median, 15–40 nmol/L) suggest vitamin D doses < 1000 IU (25 µg) daily from 27 weeks’311 and from 12 weeks’303,305 gestation are inadequate to ensure vitamin D levels > 50 nmol/L in late pregnancy, although doses of 1000 IU (25 µg) daily have achieved these levels in a small study of pregnant women with low vitamin D.309 There are several poor-quality trials of high-dose vitamin D supplementation (doses of 120 000–600 000 IU [3000–15 000 µg] immediately) during pregnancy,145,306,310,320 which do not provide adequate evidence to support intermittent high-dose vitamin D during pregnancy in clinical practice. The best study to date showed doses of 4000 IU (100 µg) daily were both safe and effective at increasing serum 25(OH)D > 80 nmol/L in all women and their neonates, regardless of race.41 No hypercalcaemia or hypercalciuria occurred.
Recent studies have suggested low vitamin D (< 50 nmol/L) is common in pregnant women in Australia, with reported prevalence figures of 48% in Sydney,327 26% in Campbelltown, NSW,328 35% in Canberra328 and 26% in rural Victoria.235 An older study found 80% of pregnant dark-skinned and/or veiled women in Victoria had levels < 22.5 nmol/L.29 These prevalence figures are important; if vitamin D doses of < 1000 IU (25 µg) daily are inadequate to achieve vitamin D > 50 nmol/L, the US Institute of Medicine (IOM) recommended daily allowance (RDA) for pregnancy of 600 IU (15 µg)99 may not be an actual RDA sufficient to meet or exceed requirements for 97.5% of the population.
Given the high prevalence of low vitamin D in pregnant women and the potential adverse effects on fetal bone health, and emerging evidence on other pregnancy outcomes, it is not unreasonable to check vitamin D status in all pregnant women and supplement to achieve maternal levels > 50 nmol/L.298 Treatment should be paired with health education and advice about safe sun exposure. Further well designed prospective trials of supplementation during pregnancy addressing safety considerations are needed.292 In the longer term, an economic analysis of the costs of screening compared with supplementation without screening will be relevant.
Vitamin D needs in lactation
Lactation is characterised by net calcium loss, with temporary bone demineralisation and recovery after weaning. The combination of parathyroid hormone-related protein (PTHrP) produced by the lactating breast and low oestradiol levels stimulate skeletal resorption, with a net loss of 5%–10% of BMC over 2–6 months of exclusive breastfeeding.291
Two studies have examined serial BMD in relation to vitamin D in lactating women.329,330 One study in predominantly white women found a decrease in BMD over the period of lactation,331 while the other study of white mothers found no change in BMD over 6 months of lactation.329 Neither study found an association between BMD and maternal vitamin D status. A third study comparing BMC in breastfeeding mothers, formula-feeding mothers and controls332 found significant decreases in BMC in the spine, femur, hip and whole body in breastfeeding women that were not related to vitamin D receptor genotype or calcium intake. Other studies have noted that demineralisation during lactation is independent of calcium intake, and that increased calcium intake is associated with increased urinary calcium excretion.291 No studies have yet examined the impact of maternal vitamin D status on post-weaning recovery of skeletal mineralisation.291
Trials of vitamin D supplementation in breastfeeding women have found doses of 1000 IU daily for 6 weeks333 to 3 months304 were inadequate to raise levels to > 50 nmol/L in women with low baseline vitamin D, although 2000 IU (50 µg) daily for 3 months achieved this end point in one of these studies.304 In comparison, 2000 IU (50 µg) was inadequate to ensure levels > 50 nmol/L in completely covered women with low vitamin D levels in Saudi Arabia,334 although another study comparing 2000 IU (50 µg) with 4000 IU (100 µg) daily for 6 months in women with baseline vitamin D > 50 nmol/L found the 2000 IU dose increased maternal levels to 90 nmol/L and infant levels from 20 nmol/L to > 50 nmol/L (without other infant supplementation).335,336 A trial in breastfeeding women used maternal vitamin D doses of 6400 IU (160 µg) per day in one arm to ensure infant levels > 50 nmol/L.337 Breast milk is a poor source of vitamin D338 and, like other age groups, breastfed infants are dependent on skin synthesis for their vitamin D stores.339
There is limited evidence for the effect of vitamin D on maternal bone health during lactation and after weaning, and inadequate evidence to recommend a higher target level for breastfeeding mothers than the current target level for healthy adults of > 50–60 nmol/L. There is also currently inadequate evidence to support maternal vitamin D supplementation as a single strategy to treat low vitamin D levels in exclusively breastfed neonates.
Vitamin D needs in infants, children and adolescents
Vitamin D is important for bone health and muscle function throughout childhood and adolescence. Adequate vitamin D status is required to prevent rickets and to promote normal bone growth and mineralisation as peak bone mass is acquired.
Rickets is a generalised disruption of skeletal mineralisation (osteomalacia), together with abnormal growth plate mineralisation and development during periods of linear growth. Rickets occurs most commonly in infancy, although it is also seen in adolescents.340 Most rickets in childhood is due to low vitamin D, although there is no absolute 25(OH)D level associated with rickets. Low calcium and/or phosphate intake or increased losses of phosphate and/or calcium may be additional contributors, or the primary cause. Case series of children with rickets from Australia,341,342,343 the US344,345,346,347,348 and Canada349,350,351 have noted almost all affected children have dark skin and prolonged breastfeeding. In the three Australian series, 75%–95% of the affected children were migrants or born to immigrant parents.341,342,343 In New South Wales, reported cases doubled from 17 cases in 2002 to 35 cases in 2003 and were almost exclusively in recently immigrated children, or first-generation offspring of immigrant parents, from the Indian subcontinent, Africa and the Middle East.343
Seventeen studies of cohorts of infants and young children with rickets (sample size, 5–129) have reported a mean/median level of 25(OH)D < 37.5 nmol/L (12 studies ≤ 20 nmol/L),342,343,352,353,354,355,356,357,358,359,360,361,362,363,364,365 while seven case–control studies in young children with rickets (number of cases, 9–129) found a mean/median 25(OH)D level of 8–38 nmol/L, compared with a mean/median of 44–90 nmol/L in the respective controls.357,359,360,363,364,365,366 In adolescents with rickets, two studies (sample size, 15–16) have reported a mean/median 25(OH)D level ≤ 18 nmol/L,367,368 and a case–control study found a mean 25(OH)D of 13 nmol/L among 16 cases, compared with 46 nmol/L in the controls.367 In three cohorts of young children from Nigeria, South Africa and India with rickets due to low calcium intake (sample size, 14–24), the mean 25(OH)D level was 45–50 nmol/L.356,367,369 There are several other intervention trials in cohorts of children with rickets where baseline vitamin D status is not reported.370,371,372,373,374
Healing of rickets is reported with both daily dosing352,353,354,356,358,366,375 and high-dose oral362,367,368,372,374 and intramuscular355,361,371,373 vitamin D regimens. The daily vitamin D regimens reported range from 400 IU (10 µg) per day for 16 weeks352 to 1700–4000 IU (42.5–100 µg) per day for 8–14 weeks353,358 and 5000–6000 IU (125–150 µg) per day for 3–4 weeks.366,375 The high-dose oral vitamin D regimens reported range from 20 000–50 000 IU (500–1250 µg) per day for 20–30 days368,374 to single doses of 150 000–300 000 IU (3750–7500 µg)362 and 600 000 IU (15 000 µg).362,367,372,374 The immediate-dose intramuscular vitamin D regimens reported range from 150 000 IU (3750 µg)376 to 300 000 IU (7500 µg)373 and 600 000 IU (15 000 µg).355,361,371 Unfortunately, many of these trials are poor quality. In several trials, it is not possible to determine the vitamin D formulation used,362,366,367,368,371,373 while in other trials, there was no early measurement of calcium status355,356,361,367,368,371,376 or vitamin D levels were not measured after treatment.358,362,366,367,371,372,373,374 Historically, vitamin D in doses of 400 IU (10 µg) per day (the amount contained in a teaspoon of cod liver oil) has been shown to prevent rickets.326
Vitamin D status is related to other measures of bone turnover and bone health in children. There are paediatric data to suggest stabilisation of PTH occurs at 25(OH)D levels of 65–90 nmol/L,377,378,379,380 and elevated PTH is seen at 25(OH)D levels < 40–60 nmol/L.380,381,382,383,384,385,386 However, there are difficulties with this approach, as the interplay between vitamin D levels and dietary calcium intake in maintaining PTH suppression, and the effect of PTH suppression on bone development in the growing skeleton, is unclear.
There are variable results from studies examining the relationship between vitamin D, BMD and BMC in infants and adolescents, and a lack of similar studies in children.
An observational study in term infants found 25(OH)D levels were positively correlated with whole-body BMC,387 although two case–control studies in infants found 25(OH)D was not related to lumbar spine BMC and BMD.388,389 Two small RCTs of vitamin D supplements (400 IU [10 µg] per day) in breastfed infants found no difference in radial BMC between groups at 6 months.390,391
Low 25(OH)D (< 25–40 nmol/L) is associated with reduced forearm382,385 and tibial385 BMD in female adolescents, and a positive association has been found between 25(OH)D and BMD at the spine, femoral neck, and radius, as well as radial BMC in adolescent girls.392 In a 3-year prospective study of adolescent girls, baseline 25(OH)D status correlated positively with change in lumbar spine BMD and bone mineral apparent density (BMAD) and femoral neck BMD over the study period.393 The difference in adjusted 3-year BMD accumulation between those with baseline vitamin D > 37 nmol/L compared with those with baseline levels < 20 nmol/L was 4%. Higher vitamin D intake was also associated with increased change in lumbar BMD over the study period.394 A study of 18–20-year-old men also found higher 25(OH)D (using the median cut-point of 44 nmol/L) was positively correlated with BMC and BMD at all sites measured (lumbar spine, femoral neck, trochanter and total hip).395 Conversely, other studies have reported no correlation between vitamin D status and BMD in Indian school children396 and 16–20-year-old women.397
The vitamin D receptor is also important in the relationship between vitamin D status and BMD in children. Vitamin D receptor gene polymorphism is associated with increased intestinal calcium absorption and increased BMC and BMD in children,398,399 and with the response to supplemental vitamin D in adolescents.400
Vitamin D supplementation in children and adolescents has not been shown to increase BMC or BMD during childhood and adolescence. A 2010 meta-analysis included six RCTs (884 participants) of vitamin D3 supplementation for 1–2 years’ duration in children aged 8–17 years.401,402 The meta-analysis found supplementation had no effect on total-body BMC or BMD of the hip or forearm, although there was a trend to a small effect on lumbar spine density and towards a larger effect for total-body BMC in participants with lower vitamin D levels. Four of the included trials used vitamin D doses of ≤ 400 IU (10 µg) per day.108,403,404,405 Supplements achieved mean 25(OH)D levels > 50 nmol/L in two trials404,405 and in the high-dose group of a third study.392 In two of the other studies included in the meta-analysis, vitamin D levels remained < 50 nmol/L in the intervention group.248,403 Two subsequent RCTs in adolescent girls with low baseline vitamin D found intermittent high-dose vitamin D supplements (achieving levels of 56–75 nmol/L) had no effect on BMD in girls post menarche,406,407 although one of the trials found a significant increase in BMC in girls within 2 years of menarche.407
Only one trial has reported on fracture outcomes392 and found no difference in self-reported incident fractures with vitamin D supplements. No trials reporting childhood vitamin D status and adult bone health outcomes have been identified.
Based on available evidence, the recommended level of 25(OH)D for infants, children and adolescents for optimal bone health remains at > 50 nmol/L. Further data are required before recommending a higher target level for bone outcomes or other health effects.
The adequate intake for those < 12 months and the estimated average requirement for vitamin D in children and adolescents is 400 IU (10 µg) daily, with a recommended dietary intake of 600 IU (15 µg) per day.99 These figures assume minimal sun exposure.
For children and adolescents with low vitamin D, supplementation may be required. The following list summarises the dose and duration to achieve mean/median levels > 50 nmol/L in children with low baseline vitamin D (mean/median < 50 nmol/L) without rickets. The recommendations below underpin the dosing table in the new paediatric position statement.
- Neonates:
- Daily regimens: 400 IU (10 µg) per day for 7 weeks,408 15 weeks,304 6 months,409,410 10 months,411 and 12 months;329 or 400 IU (10 µg) per day for 3 months in conjunction with maternal supplements of 2000 IU (50 µg) per day334 or 500–1000 IU (12.5–25 µg) per day for 3 months.412 Neonatal doses of 100–200 IU (2.5–5 µg) per day for 6 months did not achieve this end point.409,410
- High-dose regimens: 100 000 IU orally given at 0, 3 and 6 months.413
- Infants/preschoolers:
- 2000 IU (50 µg) per day or 50 000 IU (1250 µg) weekly for 6 weeks.414
- Primary school age:
- Adolescents:
- Daily regimens: 400 IU (10 µg) per day for 16 weeks,415 400–800 IU (10–20 µg) per day for 12 months,405,416 or 2000 IU (50 µg) per day for 12 months.392,400 Doses of 400–800 IU for 12 months403 or over winter393,417 did not achieve this end point in other studies.
- High-dose regimens: oral 50 000 IU (1250 µg) monthly for 12 months,418 oral 100 000 IU (2500 µg) immediately maintained levels at 2 months,419 oral 150 000 IU (3750 µg) 3-monthly for 1 year,406 intramuscular 600 000 IU (15 000 µg) maintained levels at 3 months, but not 6 months.361
A further four trials have reported on preventing the seasonal decline in vitamin D in children using high-dose therapy. In healthy primary age children, oral doses of 150 000 IU (3750 µg) at the start of winter420 or two doses of 100 000 IU (2500 µg) at the start and middle of winter421 maintain vitamin D levels > 50 nmol/L over the winter months. In healthy adolescents, three oral doses of 100 000 IU (2500 µg) 2–3-monthly384,422 maintain vitamin D levels > 50 nmol/L year round.
Vitamin D needs in healthy adults
For the purpose of this discussion, the age range for “healthy adults” is 20–65 years. Where studies included people from a wide age range, the mean age (≤ 65 years) was used to determine inclusion.
The IOM report99 compared the association between baseline or attained 25(OH)D and changes in BMD during the follow-up period. Six studies were in the age group for healthy adults:
- One study reported 25(OH)D levels predicted change in BMD (positive association).423
- Five studies reported no association between 25(OH)D and BMD282,424,425,426 or did not report any findings on this association.403
In light of these findings, it was surprising that the IOM concluded that there was “fair evidence” to support an association between serum 25(OH)D and BMD in the 18–50-years age group.
The IOM evaluated the effect of vitamin D supplementation on BMD. Twelve of these studies were in the age group of healthy adults; six reported a beneficial effect from vitamin D supplementation on BMD,17,144,427,428,429,430 while six found no effect.403,425,426,431,432,433 The daily dose of vitamin D in all of these studies was 800 IU (20 µg) or less of vitamin D3 (or the equivalent of vitamin D2), which is much lower than the level recommended today to increase 25(OH)D concentrations up to those associated with optimum health.285 In addition, most of these studies also gave calcium supplements with vitamin D. The IOM concluded, for all age groups, that both supplements are required to achieve a beneficial effect on BMD, while vitamin D by itself did not significantly increase BMD.99 It seems reasonable to conclude that this also applies to healthy adults, given that six RCTs with participants from this age group reported beneficial effects.17,144,427,428,429,430
The IOM also compared baseline 25(OH)D levels with subsequent risk of fracture.99 Since publication of these findings, at least a further seven cohort studies have been published,434 although only two were in the age range for healthy adults, and neither showed any association between baseline 25(OH)D levels and subsequent risk of fracture.435,436 However, it seems reasonable to conclude that 25(OH)D should have consistent associations across age groups, and the pooled relative risk of fracture (hip and/or non-vertebral) is 1.34 (95% CI, 1.13–1.59) comparing the lowest 25(OH)D quartile with the higher reference category in each study.434 This indicates a weak effect associated with low vitamin D status, although the possibility of residual confounding from the two most important confounders (obesity and physical activity) cannot be excluded.
At least 24 RCTs have been carried out using vitamin D (alone or with calcium).87 Meta-analyses of these studies have reported inconsistent findings, with some concluding that vitamin D is only beneficial against fractures when combined with calcium,57,87,437 and others concluding that vitamin D taken in higher doses (> 700 IU [17.5 µg] per day) is effective by itself.44,45,438,439 The observed inconsistency has arisen because many of the studies that used calcium in combination with vitamin D usually had higher doses of vitamin D (700–800 IU [17.5–20 µg] per day) compared with studies that gave vitamin D by itself (400 IU [10 µg] per day). Vitamin D doses of 400 IU (10 µg) per day are only likely to have raised 25(OH)D levels by about 10 nmol/L.440 Thus, it is not possible to conclude at present whether there is any beneficial effect on fracture incidence from vitamin D by itself. Furthermore, only two randomised clinical trials were conducted with subjects in the age range of healthy adults. Both studies were in postmenopausal women, and neither showed a reduction in fracture incidence from taking vitamin D.17,441
Vitamin D needs in older adults and individuals with osteopenia and osteoporosis
There are no data to suggest that vitamin D alone is effective in maintaining or increasing BMD. However, treatment with the combination of calcium and vitamin D prevents bone loss and results in small increases in BMD at most sites.85,351 The addition of vitamin D to calcium is also likely to reduce the risk of falling, particularly in winter, in patients with a history of falling and vitamin D insufficiency (serum 25[OH]D < 60 nmol/L).442,443 A 2009 meta-analysis suggests vitamin D supplementation decreases falls incidence by 19% in older individuals with a history of vitamin D deficiency when treated with daily doses > 700–800 IU/day and when serum 25(OH)D levels are increased > 60 nmol/L.443
Although some individual studies show primary fracture risk reduction with vitamin D alone,86 the overall evidence from several meta-analyses shows no effect of vitamin D treatment alone on fracture risk.87,44 Evidence that vitamin D reduces the risk of non-vertebral and hip fractures is most compelling with the use of additional calcium.11,44 Large annual doses of vitamin D are not recommended to either treat vitamin D deficiency or to prevent fractures. In addition, the safety of high-dose vitamin D supplementation warrants further study, as the post-dose levels of 25(OH)D seen in one study using 500 000 IU (12 500 µg) — which achieved serum levels of ≥ 120 nmol/L — may have had detrimental effects on falls and fractures in older women.214 In this regard, studies using either monthly vitamin D3 doses of 50 000 IU (1250 µg)223 or a loading dose of 10 daily doses of 50 000 IU (1250 µg) vitamin D3444 achieved more modest increases in serum 25(OH)D to just above the optimal target range (75 nmol/L) at 3 months.
Vitamin D alone may reduce incidence of primary fractures for those who have inadequate serum levels of 25(OH)D, particularly in institutionalised patients, and also when combined with calcium supplements.11,88,89,445 In women and men aged > 50 years, the combination of vitamin D with calcium, but not vitamin D alone, had a modest effect in preventing fractures (relative risk reductions of 13%–24%), particularly in those with long-term compliance rates ≥ 80%.11 The daily dose of vitamin D should be at least 800 IU (20 µg), with larger monthly doses of 50 000 IU (125 µg) being an alternative.
Despite the limitation of poor adherence in two studies examining the effects of vitamin D or calcium, either alone or in combination, there is no evidence that they are effective in reducing fractures in older women and men with pre-existing minimal trauma fractures.13,87 In these individuals, anti-osteoporotic drugs should be used instead. In women treated with commonly used antiresorptive drugs, treatment responses are improved in those with optimal serum 25(OH)D levels. In a study of women with postmenopausal osteoporosis treated with antiresorptive drugs, vitamin D-deficient women (defined as < 20 ng/mL or 50 nmol/L) had lower increases in annualised spine and hip BMD. Also, fracture incidence was higher among the women with deficiency.446
Thus, a target serum level ≥ 50 nmol/L should be aimed for in women and men taking antiresorptive drugs to optimise skeletal responses. Most patients require 800–2000 IU (20–50 µg) vitamin D per day to achieve these levels.447 In addition, serum levels ≥ 50 nmol/L will minimise the risk of hypocalcaemia following bisphosphonate therapy,57,448 and may reduce the severity of the acute-phase reaction commonly seen after the first intravenous infusion of zoledronic acid.449
The recent IOM report43 concluded that vitamin D deficiency was defined as 25(OH)D < 50 nmol/L. From meta-analyses of vitamin D supplementation for falls and fracture prevention, the serum 25(OH)D thresholds are 60 nmol/L and 75 nmol/L, respectively. Thus, in Australia it seems prudent to aim for serum concentrations of 25(OH)D of at least 50 nmol/L at the end of winter or 60 nmol/L in summer for optimal bone health. To aim for this target level of at least 50 nmol/L at the end of winter, most Australians will require between 800 IU and 2000 IU (20–45 µg) of vitamin D3 per day. A recent US Endocrine Society guideline was consistent with this and recommends that all adults aged 50–70 years and > 70 years will require at least 600–800 IU (15–20 µg) of vitamin D3 per day to maximise bone health and muscle function.46 However, to raise the serum level of 25(OH)D above 75 nmol/L, as both the Endocrine Society46 and the International Osteoporosis Foundation47 recommend, may require at least 1500–2000 IU (37.5–50 µg) per day of supplemental vitamin D; doses of up to 10 000 IU (250 µg) per day have proven to be safe.46
Other nutritional influences on bone health
The role of proteins, minerals and vitamins in bone health
Protein
In older adults, adequate dietary protein contributes to the maintenance of bone health,123 although it is unclear whether the source of the protein (animal or vegetable) is a major factor in determining the effect. Protein is an important component of bone, and higher protein intakes have been associated with reduced risk for hip fracture126,127 and greater bone density.123,124 Protein requirements are increased in older people by about 20%, and adequate protein is important in minimising bone loss and facilitating calcium absorption.4 Supplementation with a high-protein drink after hip fracture has been found to reduce bone loss and the length of hospitalisation.450 With the exception of frail older people on inadequate diets, most Australians consume sufficient protein, with many consuming protein excess to their dietary requirements.
Minerals
Other nutrients may be of biological significance to the development and maintenance of bone. These include phosphorus, sodium, potassium, magnesium and zinc.
Phosphorus does not seem to influence skeletal homoeostasis within normal ranges of intake, although excessive intakes, particularly when combined with low calcium intake, may be harmful.451 Foods that are high in phosphorus are milk products, animal protein foods (eg, poultry, fish, meat and eggs), as well as grains and legumes.
A low-potassium diet increases urinary calcium losses, while a high-potassium diet reduces this. Conversely, a high-sodium diet increases urinary calcium losses, and reducing salt intake reduces urinary calcium excretion.452 A higher intake of sodium has also been associated with lower bone mineral density (BMD).453,454,455
Several studies in older adults have examined the effect of a diet high in fruit and vegetables on BMD;130,132,456 a dietary pattern high in fruits and vegetables — which are rich sources of potassium — has a beneficial effect on BMD. It has also been hypothesised that dietary “acid load” contributes to increase systemic acid load and net acid excretion, thereby increasing bone loss leading to osteoporosis. However, a recent systematic review and meta-analysis found no evidence that an alkaline diet was protective of bone health.116
Magnesium is primarily found in bone (50%–60%). Dairy products, fruits, vegetables and whole grains are good sources of both potassium and magnesium. Dietary magnesium interacts with dietary calcium and potassium to influence absorption and retention of calcium. Magnesium supplementation (1830 mg/day) for 30 days in postmenopausal women with osteoporosis may reduce bone turnover.457
Zinc is essential for growth and is required for the growth, development and maintenance of healthy bones. Zinc has been demonstrated to have a stimulatory effect on bone formation and mineralisation. Bone growth retardation is seen in conditions associated with zinc deficiency.
In humans, the primary biological functions of copper, manganese and boron do not appear to be bone metabolism and maintenance of skeletal integrity. A severe deficiency of copper will affect bone, but intervention studies have reported inconsistent findings.458,459,460
Vitamins
Vitamin K is a fat-soluble vitamin required for bone metabolism, including osteoblastic osteocalcin formation.461,462 There are two main forms of vitamin K. Vitamin K1 is present in dark green leafy vegetables, fruits, and vegetable oils, with small amounts also being found in dairy products and grains. Vitamin K2 is found in fermented dairy and soy products, fish, meat, liver and eggs. Vitamin K is not associated with increased BMD at the femoral neck, but is associated with increased BMD at the lumbar spine.463
Vitamin A is a fat-soluble vitamin that is involved in bone remodelling. There are different types of vitamin A in the diet and in supplements: retinol is found in liver and animal products (eg, dairy foods and eggs), and β-carotene and other carotenoids are found in fruits and vegetables. Excess retinol may be detrimental to bone health at high intakes.464,465 However, there is no evidence of any association between β-carotene intake and osteoporosis or related fractures, indicating that there is no risk from consuming large amounts of fruits and vegetables rich in β-carotene.
Vitamin C plays an essential role in bone collagen synthesis. Fruits and vegetables are good sources of vitamin C; vitamin C is particularly found in citrus fruit and juices, peppers, broccoli, tomato products and green leafy vegetables. Epidemiological studies show a positive association between vitamin C and maintenance of bone mass.133,466,467
The role of exercise
Regular physical activity and exercise is recognised as one of the most effective lifestyle strategies to maximise peak bone mass during growth. Exercise also has a role to play in the prevention of bone loss during ageing. However, the osteogenic benefits of exercise are dependent on the stage of life and the relative risk of fracture. There is strong evidence that growing bone has a greater capacity to adapt to increased loading (weight-bearing exercise) than mature bone.468 Thus, it has been suggested that childhood and adolescence may represent the optimal “window of opportunity” in which exercise can improve bone strength and protect against osteoporosis and fragility fractures in old age — if these exercise-induced skeletal benefits are maintained into later life. Indeed, it has been reported that a 10% higher peak bone mass can delay the development of osteoporosis by 13 years and reduce the risk of fracture by 50%.50,51 For this reason, there has been considerable interest in quantifying the effects of exercise on bone accrual during growth and defining the appropriate mode, intensity, frequency and duration of exercise, in addition to the precise timing of exercise (childhood or adolescence), necessary to optimise bone health early in life.
The influence of exercise on bone
Exercise effects on bone mass and density
There is robust evidence9,10,11,12,13,14,15,16,17,18,19,20,21 that children who participate in moderate- to high-impact weight-bearing physical activity interventions experience greater gains in bone mineral content (BMC) and bone mineral density (BMD) at clinically relevant sites compared with less active controls.109,110,469,470,471,472,473,474,475,476,477,478,479,480 Most of these trials included targeted bone loading activities (eg, jumping, skipping, hopping, running, aerobics, ball games and strength training) and involved either extra physical education classes or were additional to normal physical education and implemented before or after school hours for 3–50 minutes per session, 2–5 times per week for 3–36 months. Overall, the exercise-induced gains in BMC and BMD typically ranged from 1% to 6% in both boys and girls, with the greatest improvement seen at the femoral neck.
Exercise effects on bone structure and strength
Whether exercise can enhance bone size and geometry during growth, which are independent determinants of whole bone strength, is less certain. However, this is an important clinical question because small changes in the structure and internal architecture of bone can significantly increase the mechanical strength of bone independent of marked changes in bone mass.481 Advances in non-invasive bone imaging techniques (peripheral quantitative computed tomography [pQCT], magnetic resonance imaging [MRI] and dual energy x-ray absorptiometry [DXA]-based hip structural analysis [HSA]) have made it possible to quantify bone structural adaptations to loading and estimate the effect of exercise on whole bone strength. In young athletes involved in weight-bearing sports that generate moderate- to high-impact loads (eg, gymnastics, ballet, tennis), there is compelling evidence that exercise during growth can significantly increase the size, structure and strength of bone.482,483,484,485,486,487 In contrast, the findings from a limited number of randomised controlled trials (RCTs) that evaluated the effect of exercise interventions on bone structure and strength using pQCT, MRI or HSA are less consistent.471,475,478,479,488,489 This is highlighted by the results from a recent systemic review and meta-analysis that reported a small but significant effect on lower extremity bone strength with exercise in prepubertal and early pubertal boys (effect size, 0.17; 95% CI, 0.02–0.32) but not in prepubertal girls or adolescent boys or girls.490 However, these findings should be interpreted with caution because only five studies were included in the analysis and there was considerable heterogeneity in terms of the type and dose of exercise prescribed, the study duration (7–24 months) and number of participants in each trial. Further long-term and adequately powered trials are still needed to address whether exercise can enhance the structure and strength of bone during growth.
Are the benefits of exercise on bone during growth maintained into later life?
An important clinical question that remains unanswered is whether the exercise-induced skeletal benefits attained during growth are maintained into adulthood and reduce the risk of fracture later in life. There is some evidence from studies of retired athletes that indicates BMD gains during growth may be maintained for up to 20 years.491 Similarly, the findings from an 8-year follow-up to a school-based exercise intervention showed that hip BMC was still significantly higher (1.4%) in children from the intervention compared with the control group.492 However, limited data in older retired athletes suggest that the effects on bone mass are largely eroded over time,493 although there is some evidence that exercise-induced benefits in bone size and structure may be permanent.494 In terms of fragility fractures, retrospective studies in former athletes examining fracture incidence have produced equivocal findings.493,494,495,496 Given the long time interval between exposure (exercise during growth) and outcome (fracture in the elderly), it is unlikely that we will ever have high-level evidence to confidently conclude that exercise-induced skeletal gains during growth prevent osteoporosis and fractures later in life.
The development of clinically tested exercise regimens
It is well known that the skeleton adapts to changes in mechanical loading, and that loads (strains) that are dynamic, high in magnitude, applied rapidly and in unusual or diverse loading patterns are particularly effective for stimulating an osteogenic response. In addition, relatively few loads or repetitions are needed to elicit a positive skeletal response, and separating loading exercises into discrete bouts with periods of rest appears to optimise skeletal gains. With this knowledge, most of the intervention trials in children that were successful incorporated a variety of dynamic and diverse weight-bearing activities, such as jumping, skipping, hopping, running, dancing, plyometrics, ball games and step aerobics. While it is difficult to determine from these trials which exercises are most effective, several intervention trials in children have reported positive effects on hip bone mass following relatively simple jumping programs (eg, 100 box jumps, three times per day for 7 months,470 and 10 jumps, three times each school day for 8 months497). Overall, there is compelling evidence that weight-bearing impact activities are most effective for improving bone health.
Currently, there is a lack of high-level evidence to support specific exercise prescription guidelines for improving peak bone mass. Many questions still remain as to how much, how often, and to what magnitude or how long children need to exercise to optimise bone strength. In an attempt to quantify the optimal load (intensity) needed to enhance bone accrual, several loading exercise interventions measured the ground reaction forces generated from a variety of impact exercises as a surrogate for the skeletal loads (strains) imparted on bone.110,470,475,497 These trials showed that loads ranging from three to five times body weight were effective for producing an osteogenic response,109,110,471,473,474,475,479 with some evidence that higher loads (up to 8.8 times body weight) were associated with greater skeletal gains.470
In terms of training duration and frequency, many of the effective school-based interventions prescribed weight-bearing exercise for 20–50 minutes, two to five times per week, for 8–36 months.110,491,495,498,499 However, comparable exercise-induced skeletal gains have been observed following short periods of weight-bearing exercise that involved 3–12 minutes of various jumping activities performed 3–5 days per week over 7–20 months.470,472,473,474,475,476,478,489
In summary, the available evidence from intervention trials indicates that children should engage in a diverse range of dynamic, moderate- to high-impact, multidirectional weight-bearing activities at least three times per week in order to optimise bone health. Whether there is an optimal number of loads or dose (duration) of training requires further research, but we know that school-based interventions that incorporate weight-bearing activities ranging from 20 to 50 minutes per session or more specific targeted jumping interventions from 3 to 12 minutes can enhance bone mineral accrual.
Exercise needs for children
Several lines of evidence from RCTs indicate that the skeletal responses to exercise during growth are maturity dependent. Unilateral loading studies of young female tennis and/or squash players have reported that bone mass, structure and strength are greater in the dominant playing (loaded) arm compared with the non-playing arm in those players who commenced training before or at menarche rather than after menarche.484,500 More recent data in pre-, peri- and postpubertal players suggest that the greatest skeletal benefits from exercise occurred during the prepubertal years because no further side-to-side differences were detected with advancing maturity.482,483,486
The highest quality studies suggest that, over the course of a school year, both moderate- (eg, team sports) and high-impact (jumping-related) exercises can improve bone at various skeletal sites for:
- prepubertal girls and boys (Tanner I)470,501
- early pubertal children (Tanner II–III)110,488
- late/postpubertal adolescents (Tanner IV–V).502
Improvements tend to be greatest at sites subjected to the highest load magnitudes and frequencies (lower extremities), with changes being more modest as distance from site of loading increases (spine and whole body). Consistent with these findings, the results from a comprehensive systematic review of both randomised and non-randomised exercise-controlled trials reported that exercise-induced gains in BMC and BMD, averaged over 6 months, ranged from 0.9% to 4.9% in prepubertal children and 1.1% to 5.5% in early pubertal children compared with matched controls.469 In contrast, the few intervention trials that have been conducted in postpubertal children have observed either no additional skeletal benefits following exercise498,503,504 or relatively small gains.469,488,502
Exercise has also been observed to improve paediatric bone strength by inducing changes in structural parameters (eg, cortical thickness and cross-sectional moment of inertia);470,501 however, a recent meta-analysis of bone strength outcomes concluded that exercise during growth enhances bone strength indices in boys only.490 Considerable sexual dimorphism and maturational heterogeneity exist with respect to the degree and site at which such bone structural adaptations occur during growth.482,505
Additional long-term exercise trials are required before definitive exercise recommendations can be made. Nonetheless, bone benefits can be observed in some children after even very brief exposure to exercise (3–10 minutes, 2–3 days per week) if the activity is weight-bearing in nature and of sufficient intensity (over three times body weight).488 In the absence of dose–response evidence, however, typical exercise recommendations for paediatric bone health are relatively broad: 10–45-minute bouts, 3–7 days per week.482,506 Data on the maintenance of the osteogenic benefits of childhood exercise through to adulthood are lacking, although recent prospective Australian observational evidence suggests fitness as a child does predict bone mass at age 30, even after taking current fitness into account.507 While physical inactivity increases fracture risk in children,508 it should also be noted that some exercises in children (most notably sports participation) may increase both bone mass and fracture risk, especially in boys, so there is a need to also consider the risks as well as the benefits associated with physical activity.
In summary, it appears that the prepubertal and early pubertal years represent the optimal time for exercise to enhance bone strength during the first two decades of life.
Exercise needs for healthy adults, older adults and individuals with osteopenia and osteoporosis
Age-related changes in bone density and fracture risk in relation to physical activity patterns over the life span
Differences in age-related bone mass changes are often observed between habitually active and sedentary non-athletic individuals.509,510,511,512,513 Consistent with such bone density findings, hip fracture incidence has been observed to be as much as 30%–50% lower in older adults with a history of higher levels of physical activity in daily life, compared with age-matched, less active individuals.514,515,516,517,518,519,520 Fewer data are available for men, but they are generally consistent with the findings for women. Higher physical activity level has also been linked to reduced osteoporotic fracture prevalence or incidence in older adults. For example, in the Study of Osteoporotic Fractures,519 women who reported walking for exercise had a significant 30% reduction in hip fracture risk compared with women who did not walk for exercise.
Physical activity and bone health in premenopausal women
Trials of exercise and BMD in premenopausal women have been the subject of a number of meta-analyses.52,53,521,522,523,524 Although many of the individual trials lacked statistical power to demonstrate significant treatment effects, the meta-analyses all concur that exercise has positive effects on BMD at the lumbar spine in premenopausal women. Aerobic training, high-impact training (such as jumping), resistance training, and combined aerobic and resistance programs all increase lumbar spine BMD by about 1% per year on average, relative to sedentary controls. Changes at the femoral neck or greater trochanter have been assessed less frequently in these studies of premenopausal women. However, significant changes at the femoral neck have been observed in programs that combine weight-bearing aerobic and strength training522 and high-impact aerobic (jumping/stepping) exercise.525 Significant changes to BMD at the trochanter have been observed after isolated high-impact exercise, including jumping and skipping,526 50 jumps 6 days per week,527 and jumping/lower-extremity resistance training with a weighted vest.528
The non-skeletal effects of exercise in premenopausal women may be equally important for future fracture risk and general health. A meta-analysis found that resistance training in premenopausal women resulted in significant changes in lean mass (+ 2 kg), muscle strength (+ 40%) and losses of body fat (− 2%), compared with minimal changes in the control groups.521 The most economical prescription with the broadest benefits for body composition and bone health, as well as neuromuscular function, would be resistance training as the primary exercise modality. Adding high-impact forces/movements may further enhance benefits for the femoral neck or trochanter, lower extremity muscle power, and dynamic balance.525
The physiological response in bone and muscle is proportional to the magnitude and rate of strain imposed,529 and successful programs have used intensities at the higher ranges in general. Therefore, moderate- to high-intensity progressive resistance training and/or high-impact training is recommended as the primary intensity of planned exercise in this age group. It should be noted that high-impact programs have successfully increased trochanteric BMD by 3%–4% in young women via jumps about 8 cm off the ground. This kind of jump produces ground reaction forces that are three to four times body weight (thus high impact), but are feasible for non-athletic women, are infrequently associated with injuries, and are able to be completed in about 2 minutes per day.527
Two or three days per week of weight-lifting, aerobic exercise, or high-impact programs have been shown to augment bone density significantly compared with sedentary controls if continued for at least 1–2 years. Fifty jumps of 8.5 cm height, 6 days per week over 6 months were associated with a 2.8% increase in trochanteric BMD compared with controls.527 Overall, the clinical trials literature would support a recommendation of about 40–50 jumps or repetitions of a given weight-lifting exercise per training day.
In summary, exercise programs that combine novel- or high-impact activity with high-intensity resistance training appear most effective in augmenting BMD in premenopausal women at the femoral neck and lumbar spine. High-impact-alone protocols (such as jumping) are effective only on hip BMD in this group.52 For isolated resistance training in premenopausal women, the relative BMD change for lumbar spine was almost 1%,53 whereas femoral neck BMD changes were not significant. Further RCTs of resistance training in premenopausal women of sufficiently long duration and providing optimum type, intensity and volume of loading are required.53
Physical activity for postmenopausal women and older men
Recent meta-analyses54,524,530 suggest that the beneficial effect of exercise on bone density in older adults is both modality and intensity dependent. Clinical trials of low-impact, low-intensity exercises, such as stretching, calisthenics or low-intensity weight-lifting exercise, in postmenopausal women have not been shown to significantly improve bone density compared with controls at any site.54,531 Walking alone has not been shown to significantly improve BMD at the spine or hip, or to reduce fractures in RCTs.54,530 Thus, older recommendations532 suggesting that weight-bearing exercise, such as simple walking, is sufficient for optimisation of bone health are not consistent with the current evidence base.530 It is likely, therefore, that the benefits of walking on fracture risk noted in epidemiological studies519 are multifactorial, rather than being attributable to higher bone density alone in physically active individuals.
Modality of exercise
In general, the older the individual, the more favourable resistance training appears to be. Effective resistance-training regimens have usually involved high-intensity training (70%–80% of peak capacity as the training load), which is progressed continually over the course of the intervention.521,533,534 Kohrt and colleagues535 found that both aerobic activities with high ground reaction forces (eg, combined walking, jogging, stair climbing) and exercises with high joint reaction forces (eg, weight-lifting, rowing) significantly increased the BMD of the whole body, lumbar spine, and Ward’s triangle, but that only aerobic activities with high ground reaction forces increased BMD at the femoral neck.535 The weight-lifting group preserved femoral neck BMD relative to controls, as has been seen in other resistance-training studies.534,536 However, lean mass and muscle strength increased only in the weight-lifting group. Consideration of non-skeletal risk factors for osteoporotic fracture (such as muscle weakness, poor balance, sarcopenia) favours high-intensity resistance training over high-intensity aerobic training.521,535,537,538
Intensity of resistance training
The predominant training factor that appears to influence effectiveness of exercise on bone is the intensity and novelty of the load, rather than the number of repetitions, sets, or days per week, or even the total duration of the program. A study comparing two different intensities of weight-lifting exercise in postmenopausal women539 found that 1 year of strength training at high intensity (three sets of eight repetitions) significantly increased BMD at the femoral trochanter, intertrochanteric site and Ward’s triangle, as well as the ultradistal forearm, compared with low-intensity training (three sets of 20 repetitions), which produced no significant changes in BMD at any site except the mid forearm. In healthy older men, high-intensity resistance training has been shown to increase BMD at the lumbar spine and greater trochanter compared with controls,540 similar to results in older women. One of the few studies of older men and women with physical frailty compared low-intensity home-based physical therapy with supervised high-intensity resistance training.541 The high-intensity weight-lifting group had significantly better BMD of both the whole body and Ward’s triangle compared with the low-intensity exercise group at the end of the study, again demonstrating the superior efficacy of more intensive exercise. Changes in muscle strength were correlated with changes in BMD only in the high-intensity group. A meta-analysis54 of low-intensity strength training found no benefit at any skeletal site. A randomised trial542 of postmenopausal women participating in a multimodal exercise program reported significant bone density improvements at the trochanter; the BMD changes were significantly and linearly related to total weight lifted, but not to the volume or quality of the non-resistance training components of the program. High-intensity resistance training is also more beneficial than low-intensity training for muscle strength gains and muscle hypertrophy, as well as associated functional impairments, obesity, depression and metabolic health. Accordingly, this modality is ideal as a multiple risk factor intervention strategy for older adults with multiple comorbidities.534,543,544,545,546,547,548 Thus, it is important to consider not only the optimal modality of exercise, but also the relative intensity, as skeletal and other adaptations are critically linked to the intensity of the loading (whether due to increased amount of weight lifted during resistance training, or higher ground reaction forces during aerobic or jumping activities).
Isolated high- or novel-impact exercise in older women
The wealth of data on impact exercise in children and younger women is not matched in older adults, attesting in part to the difficulties encountered in implementing this form of exercise when arthritis and other health conditions are prevalent. A study that randomly assigned postmenopausal women to heel drops (1.5 times body weight) or control conditions found no difference in BMD after 12 months, perhaps due to the smaller impact of this regimen compared with jumping.549 A subsequent study reported that the same jumping intervention (50 jumps, 6 days per week) successfully used in premenopausal527 women did not significantly improve BMD in postmenopausal women exercising for 12 months. A recent meta-analysis524 of controlled trials in postmenopausal women found that high- or novel-impact-only protocols were ineffective in increasing BMD at any site. Thus, as seen in premenopausal women, combined programs of resistive and high-impact loading (when feasible) would appear to be the most beneficial approach in older women.
Exercise for older adults with osteopenia, osteoporotic fracture and frailty
In addition to the above considerations on modality and intensity of exercise for healthy postmenopausal women, activity recommendations for the older age group with osteopenia or osteoporosis should include avoidance of forward flexion of the spine, particularly while carrying an object (eg, lawn bowling, bending over to pick up something from the floor, or doing sit-ups with straight legs). Such actions increase the risk of anterior compression fractures of the thoracic vertebrae in the presence of osteopenia. Similarly, high-risk activities or hazardous environments that may lead to falls in those with poor balance are best avoided. The rationale and benefit of high-intensity progressive resistance training for sarcopenia and its sequelae537 will likely exceed the benefits for BMD itself in this cohort.
In older men and women who have already sustained an osteoporotic fracture, exercise is extremely important to assist in recovery of function,550 improve quality of life,551 and prevent recurrent injurious falls.552 Progressive resistance training has been shown to be superior to standard physical therapy during the recovery from hip fracture in elderly patients. In addition, resistance training has been shown to be a useful adjunctive treatment for depression in the elderly, which is of importance because antidepressant medications are known to increase the risk of falls and hip fracture.553,554 A combination of resistance training and balance training may offer the best approach to rehabilitation in this setting, as it optimally targets the remediable physiological risk factors for falls, fractures and disability for older individuals with prior osteoporotic fracture.
There is a large burden of potentially treatable risk factors for mortality, frailty and recurrent injurious falls in older adults who have sustained a hip fracture.555,556,557 However, current clinical treatment pathways still focus primarily on repair and rehabilitation of the fracture itself rather than the underlying frailty.81,558,559,560 Few physical therapists prescribe robust resistance training to improve muscle strength,561 despite its recognised role in osteoporotic fracture and frailty. Poor outcomes may theoretically be improved through inclusion of robust strategies designed to target modifiable predictors of frailty. In one RCT, 1 year of high-intensity progressive resistance and balance training, combined with a targeted multifactorial intervention directed at major predictors of frailty, reduced both mortality and nursing home utilisation by more than 80% at 12 months after hip fracture.562 Additional trials are required to confirm and extend these findings.
Summary of evidence
The role of exercise with respect to osteoporotic fracture prevention and treatment is life-stage specific, localised to the site of loading, and highly modality and intensity dependent. The goal of exercise and physical activity shifts from the attainment of peak bone mass in childhood and adolescence to the optimisation of muscle and bone strength in young adulthood, and the attenuation of bone loss in the perimenopausal years. Thereafter, the focus is on the prevention of sarcopenia in postmenopausal women and, finally, on addressing risk factors for frailty and falls in older men and women, particularly impairments of balance and sarcopenia.
Habitual exercise has been found to have a relatively potent effect on BMD in epidemiological and cross-sectional investigations.510,563,564 Both weight-bearing aerobic exercise515,538,565,566,567,568,569,570 and high-impact and resistive exercises534,571,572,573,574,575 have had positive effects in experimental trials. Evidence suggests that a stabilisation or increase in bone mass in premenopausal women is achievable with either high-loading resistive,521,533,534,540,576 weight-bearing moderate-impact aerobic exercise511,512,565,566,570,577 or high-impact loading, particularly if combined with resistance training.526,578
In postmenopausal women, neither high-impact exercise alone nor low-intensity resistance training significantly improves bone density,54 but high-intensity resistance training or the combination of high-impact exercise and high-intensity resistance training is effective.54,578 These effects on bone density (differences of 1%–2% per year associated with exercise) may be important for both the prevention and treatment of osteoporosis and related fractures and disability, as reviewed in several recent meta-analyses.522,523,579,580,581 Walking alone in postmenopausal women improves bone density by a small amount of questionable clinical relevance,530 and these effects at the lumbar spine or the hip in the most recent meta-analysis are heterogeneous and not statistically significant. In general, because the effects of muscle contraction on bone appear to be primarily regional (ie, electromagnetic field stimulation of osteoblast function) rather than systemic, it is advised that muscle groups connected to bones of relevance to osteoporotic fracture be emphasised in exercise programs (eg, spinal extensor muscles, hip abductors, hip extensors, knee extensors, knee flexors), as well as those related to gait and balance (eg, ankle plantar flexors and dorsiflexors, inverters and everters, hip abductors).
In frail and very elderly adults, little is known about the effects of exercise on bone density itself. However, resistance training and balance exercises in combination reduce falls and risk factors for frailty, including sarcopenia, poor balance, gait instability, depression, fear of falling and cognitive impairment. When prescribed with multidisciplinary geriatric care, these interventions have been shown to improve outcomes after hip fracture, such as functional dependency and mortality.562
An emerging body of evidence suggests that multimodal exercise — inclusive of weight-bearing/high-impact/high-intensity resistance exercise — significantly reduces overall fracture risk.54 By contrast, single modality exercise of any type does not appear to reduce fracture risk,54 with the possible exception of spinal-extensor muscle resistance training, which has been shown to significantly reduce thoracic vertebral fracture incidence.54,55 Additional data are needed in men, and on fracture prevention, as is refinement of the exercise prescription for bone health and fracture treatment. For various cohorts (eg, male/female, those with osteopenia/osteoporosis), much more needs to be known in terms of the optimal modality, dose, frequency and intensity of activity to be recommended.
Other considerations in the maintenance of healthy bones
Role of antiresorptive and anabolic agents in the maintenance of healthy bones
The management of osteoporosis has several stages, all of which are focused on prevention. A healthy lifestyle, with adequate physical activity, adequate nutrition, and avoidance of excessive alcohol and smoking, is considered important in optimising peak bone mass and in helping maintain healthy bones in later life. However, many people have medical conditions and require treatments that also contribute to relatively poor bone health.582,583,584 In addition, a major part of the risk of osteoporosis is related to heritable factors.585 Indeed, evidence from twin and family studies indicates that roughly 75% of measured differences in bone density are directly inherited. Thus, many people reach older age with good bone density despite a less than ideal lifestyle. Perhaps more importantly, many people reach older ages with poor bone health, despite having maintained a healthy lifestyle.
An important aspect of osteoporosis management is that the peak of fracture prevalence is in the relatively young old; that is, it occurs before the age of 75 years.586 While each individual’s risk of fracture increases with advancing age, the overall number of individuals decreases with age, so the overall fracture prevalence then declines.
In addition to encouraging a healthy lifestyle throughout life, it is therefore critical to focus on offering appropriate treatment to people who are at increased fracture risk. The Garvan Institute Fracture Risk Calculator (http://garvan.org.au/promotions/bone-fracture-risk/calculator) has been validated in both men and women internationally. The Garvan Institute Fracture Risk Calculator takes into account age, sex, bone mineral density (BMD) (if available), prior fracture history, and falls within the previous 12 months to estimate an individual’s absolute risk of hip fracture and of other fragility fractures in the next 5 and 10 years. This calculator can and should be used as a starting point for discussions with an individual about his or her choices of treatment. An alternative risk calculator is the Fracture Risk Assessment Tool (FRAX; http://www.shef.ac.uk/FRAX/tool.aspx?country=31), which was developed by the World Health Organization and also has been calibrated using an Australian population cohort.587
In individuals at high fracture risk, especially those who have already had previous fractures, there is no doubt that specific anti-osteoporosis therapy is warranted. These treatments are not perfect but they have been shown to be efficacious — about halving subsequent fracture risk — and are well tolerated.56,57,58,59,60,61,62,63,64,65,66 The various treatments discussed below have been evaluated in placebo-controlled randomised controlled trials (RCTs) with fracture end points. However, they have not been compared head-to-head with fracture end points in RCTs.
In Australia, these treatments are covered by the Pharmaceutical Benefits Scheme (PBS) for both men and women after fragility fracture, as well as for those at high risk, without prior fracture, on the basis of age and BMD T score.67
Hormone therapy
In women, bone loss accelerates with the onset of menopause, and postmenopausal hormone therapy — oestrogen or oestrogen plus progestin — at this time will prevent this bone loss or at least minimise it. RCTs have shown that oestrogen therapy with or without progestin significantly reduces both vertebral and hip fractures.588,589 Postmenopausal hormone therapy is most suitable for the recently menopausal woman; particularly for those with menopausal symptoms and more particularly for those with an early menopause. This therapy is not recommended for postmenopausal women presenting with osteoporosis without menopausal symptoms and more than 10 years past the menopause. Bone density can be maintained with small doses of oestrogen, less than conventional doses,590,591 but bone loss will resume when treatment is stopped.
Whether used for the prevention of osteoporosis or for menopausal symptoms, women should be fully informed about the data for hormone therapy, as it is known that the risk of breast cancer is increased from long-term use of continuous combined oestrogen plus progestin therapy and that there is an increased risk of thrombotic disease with oral preparations. Cardiovascular risk is not increased when therapy is started within 10 years of the menopause.592,593
Antiresorptive therapy
The major component of antiresorptive therapy is the bisphosphonates; these are now available in multiple formulations: oral weekly (alendronate and risedronate), oral monthly (risedronate), and intravenous annually (zoledronic acid).
The oral preparations are poorly absorbed (< 1% of the dose) even when taken correctly (ie, fasting with plain water and waiting half an hour to 1 hour before breakfast).594 More recently, one of the oral preparations (risedronate) has become available in an enteric-coated weekly formulation that includes some ethylenediaminetetraacetic acid (EDTA).595 The major advantage of this new formulation is that it can be taken with breakfast, although not at the same time as calcium supplements; this may facilitate its correct usage and thus ease issues with patient adherence, just as the weekly and monthly preparations were major advances over the daily formulations. The intravenous annual formulation has the additional advantage of direct recording and observation of use and thus improved adherence.
A new antiresorptive therapy is the human antibody against the osteoclast growth and survival factor, anti-RANK ligand (denosumab). This is given as a subcutaneous injection every 6 months.
In large-scale, multinational pivotal studies, each of these antiresorptive agents has been shown to reduce the risk of vertebral fractures by 50%–70%, severe or multiple vertebral fractures by up to 90%, hip fractures by around 40%, and other non-spine, non-hip fractures by around 30%.56,57,58,59,60,61,62,63,64,65,66 It is worth noting that in some of the RCT studies, the use of antiresorptive agents has been associated with reduced mortality that is not explained by reduced fracture risk per se.62,596
However, use of these agents has been associated with side effects.597 The most common, although still not frequent, are upper gastrointestinal symptoms with oral bisphosphonates. These symptoms are generally mild but their possibility should alert the practitioner with respect to patients with known pre-existing gastrointestinal problems. On the other hand, the use of H2-receptor antagonists does not interfere with bisphosphonate absorption.
The intravenous bisphosphonates are associated with an acute flu-like illness for a few days in about one in four individuals. However, this can be largely controlled with oral antipyretic analgesics and, even if it does occur, is usually less severe with subsequent infusions. Intravenous bisphosphonate use also requires both adequate vitamin D levels, to minimise risk of transient hypocalcaemia, and adequate renal function, as transient deterioration of renal function has been reported. It makes sense to minimise this risk in older individuals by ensuring adequate hydration and slowing the infusion rate if concerned.598
The anti-RANK ligand antibody denosumab has been associated in one study with more skin infections; this was not seen in a longer extension of the same study.598 There is also a theoretical concern about its use with other biological agents (eg, for rheumatoid arthritis).
Longer-term use of potent antiresorptive agents, both bisphosphonates and denosumab, has been associated with osteonecrosis of the jaw.599,600 This has very largely been reported in the treatment of individuals with actual or potential skeletal metastases from malignancy, where the doses used are much greater (10–20-fold). In osteoporosis treatment, cases of osteonecrosis of the jaw may occur but are very uncommon and are generally much milder forms than in cancer treatment scenarios or are associated with dental procedures. These milder forms of this condition have also been noted to heal even while antiresorptive treatment was continued.
More recently, atypical fractures have been reported after long-term use of bisphosphonates for osteoporosis; no causal relationship has been proven and reports have been associated with all the bisphosphonates, including alendronate, risedronate and zoledronate.601 In one of the best datasets from Kaiser Permanente, the incidence of these atypical fractures only started to increase after about 8 years of use and was then about 78 events per 100 000 person-years of treatment.602
Non-antiresorptive agents
These include two agents, one specifically anabolic, teriparatide, and one agent with effects on both formation and resorption markers, strontium ranelate.
Based largely on its cost, teriparatide has been restricted in use on the PBS to those with “treatment failure”; that is, patients with further fractures after at least a year of effective antiresorptive therapy or patients with severe osteoporosis and intolerance to antiresorptive therapy. The studies of this agent were cut short due to osteosarcoma development in an animal safety model; this has not currently been reported in humans. In the initial shortened studies, teriparatide effectively reduced non-vertebral fractures in women and non-vertebral fractures in both men and women. It is well tolerated, with the most common adverse effect being leg cramps.598 Importantly, after the 18-month (one in a lifetime) course of teriparatide therapy currently approved, the accrued benefits will be lost if antiresorptive therapy is not instituted.
Strontium ranelate is taken once a day, usually before bedtime, and away from food or mineral supplements. It is generally well tolerated, although some patients note transient diarrhoea or skin rashes. The risk of deep venous thrombosis is also slightly increased. It has been shown to be effective in reducing fragility fractures (spinal and non-spinal) in postmenopausal women with osteoporosis. As a prespecified study end point, strontium ranelate has been shown to have efficacy in older women and has demonstrated similar efficacy in men in registration studies.598
Drug “holiday”
At times, it has been suggested that a treatment “holiday” can be offered to patients after 5–10 years of treatment with antiresorptive therapy. This is largely based on a single small study that showed some retention of benefit after 5 years of treatment.68 However, although the report stated there was no increase in non-vertebral fractures off treatment, it also noted there was a significant increase in clinical (ie, symptomatic) vertebral fracture events. Moreover, the women in this study had BMD levels at which we would not generally recommend treatment at all in Australia.
Each of the antiresorptive agents has some extension of benefit after cessation of administration that ranges from 6 months (denosumab) to up to a year or so (with a longer exposure to the bisphosphonates).
Despite the persistence of benefit, there is no evidence to support a drug holiday in individuals with severe osteoporosis. If a drug holiday is planned, a plan must also be put in place to review the patient regularly. It seems prudent to reinstate therapy if there is any further bone density decline, which is usually preceded by an increase in bone turnover marker levels.
In summary, there is a range of effective treatments that are well tolerated, with good safety profiles. Masterly inactivity on the behalf of the practitioner and not initiating treatment to reduce the risk of future fracture events is no longer justifiable in any person with a fragility fracture.
Bone density testing — what is ideal?
Until the latter half of the 20th century, the clinical diagnosis of osteoporosis was based on the use of conventional radiography, which is insensitive to bone loss or the development of an osteoporotic fracture. This approach resulted in late diagnosis and the consequent economic costs of fracture management. Over the past four decades, a number of techniques have been developed that provide an unprecedented ability to assess bone strength, most commonly quantified using the surrogate of BMD. These techniques allow the diagnosis of osteoporosis at an earlier stage, before fracture, with the result of being able to implement cost-effective antifracture therapies. These techniques also allow the monitoring of bone loss and treatment responses when BMD is remeasured after 2 years. The techniques in common clinical use are dual energy x-ray absorptiometry (DXA) and quantitative computed tomography (QCT). Other technologies available include peripheral QCT (pQCT) and, more recently, high-resolution microcomputed tomography;603 while these techniques hold great promise, they are currently mainly used in research and will not be discussed here. Similarly, magnetic resonance imaging to image bone microstructure at high resolution remains a research tool for specialised centres. Quantitative ultrasound is an interesting technology that has utility in fracture risk prediction;604 however, due to its inability to assess central skeletal sites, lack of standardisation and the lack of evidence for the use of results in guiding therapy, it is not used extensively in mainstream medicine.
Dual energy x-ray absorptiometry
Dual energy x-ray absorptiometry, due to its safety, low cost and ease of access, is now generally accepted as the gold standard for measuring BMD (g/cm2). It has high reproducibility and a low radiation dose of approximately 5 µSv.604 In clinical practice, the regions most commonly measured using DXA are the lumbar spine and the proximal femur. In the latter, only measurements of the transcervical neck of femur and the total proximal femur regions are recommended for clinical use.69 The interpretation of BMD depends on comparison with reference ranges, which are used by the DXA scanners to generate T scores (a comparison to the young normal mean) and Z scores (a comparison to the age-matched mean). Differences in reference ranges between DXA machine manufacturers have in the past caused problems,605,606,607 but this has been partly overcome in Australia by the widespread, but not universal, adoption of the Geelong reference ranges.608 A third skeletal site, the mid-shaft of the radius (1/3 site), is also used in clinical practice but is hindered by a lack of conversion equations that would allow standardisation of reference ranges across DXA manufacturers.
The clinical utility of DXA to guide therapy is well documented in numerous drug trials that have demonstrated that, in patients chosen on the basis of DXA-derived low BMD, therapy can significantly reduce the risk of subsequent fracture.609
Quantitative computed tomography
Quantitative computed tomography uses conventional CT scanners to derive volumetric bone mineral density (vBMD) (g/cm3) at skeletal sites of interest, typically the vertebral column and the proximal femur.610 The WHO BMD definition of osteoporosis is not applicable to QCT-derived vBMD, and this has hindered a standardised approach to interpretation of the results. More recently, however, software allowing the conversion of proximal femoral volumetric data (g/cm3) into the areal BMD of DXA (g/cm2) has allowed the use of DXA-derived reference ranges and the application of the WHO diagnostic criteria for osteoporosis and osteopenia.611
A number of cross-sectional studies have demonstrated the utility of QCT in distinguishing osteoporotic individuals from normal controls.612,613 However, there are very few longitudinal studies demonstrating the utility of QCT-derived vBMD to predict fracture risk and, to date, these have not demonstrated any superiority to DXA.614 There are also no large drug trials demonstrating the utility of QCT in guiding therapy in osteoporotic patients. One other major issue of QCT is radiation dose. While the radiation dose of spinal QCT is relatively low at about 50 µSv (compared with a smaller 5 µSv for DXA),604 in the proximal femur, QCT results in a significantly higher radiation dose than DXA, typically in the range of 500–1000 µSv.615
Due to the above limitations, QCT is currently less used than DXA in the management of osteoporosis.
Recommendation
The current ideal is the use of DXA to assess fracture risk by measuring lumbar spine and proximal femoral BMD in all high-risk individuals.69 In addition, the use of DXA to screen asymptomatic individuals may be worthwhile at age 65 or 70 years.70,71 Currently, Australian Medicare funds this approach in subjects over the age of 70.
The clinical utility of DXA and QCT in Australia is hampered by the lack of adequate training of many technologists performing the scans. While there are high-quality training courses available, many radiographers and nuclear medicine technologists licensed to perform bone densitometry have limited training. In addition, reporting of DXA scans is undertaken by a wide range of specialists, and while many have undertaken dedicated courses, a large number of specialists in the field have never undertaken specific training.
The ideal use of DXA and QCT would be enhanced by:
- adoption of standardised reference ranges across Australia
- adequate training and accreditation of all bone density technologists
- adequate training and accreditation of all reporting medical specialists
- adoption of absolute fracture risk assessments into the medical treatment models
- confining the use of QCT to specialists managing patients with osteoporosis.
Falls prevention
The Australian and New Zealand Society for Geriatric Medicine (ANZSGM) develops policy and practice relevant to geriatric medicine. On the subject of falls prevention, the ANZSGM recognises the 2010 update of the American Geriatrics Society and British Geriatrics Society clinical practice guideline Prevention of falls in older persons.616 This guideline makes the following recommendations with respect to screening and assessment of falls for older people:
- An older person who reports a fall should be asked about the frequency and circumstances of the fall(s).
- Older individuals should be asked whether they experience difficulties with walking or balance.
- Older persons who present for medical attention because of a fall, report recurrent falls in the past year, or report difficulties in walking or balance (with or without activity curtailment) should have a multifactorial fall risk assessment.
- Older persons who cannot perform or perform poorly on a standardised gait and balance test should be given a multifactorial fall risk assessment.
- Older persons who report a single fall in the past year should be evaluated for gait and balance.
- Older persons who have fallen should have an assessment of gait and balance using one of the available evaluations.
- Older persons who have difficulty or demonstrate unsteadiness during the evaluation require a multifactorial fall risk assessment.
- Older persons reporting only a single fall in the past year and reporting or demonstrating no difficulty or unsteadiness during the evaluation do not require a fall risk assessment.
- A clinician (or clinicians) with appropriate skills and training should perform the multifactorial fall risk assessment.
- The multifactorial fall risk assessment should include the following:
A. Focused History
[…]
B. Physical Examination
[…]
C. Functional Assessment
[…]
D. Environmental Assessment
[…]
This section provides a summary of falls risk assessment and fall prevention strategies for older people in the community, hospitals and residential aged care facilities (RACFs). Detailed information on these topics is provided in the Preventing falls and harm from falls in older people: best practice guidelines for Australian residential aged care facilities, 2009617 and Preventing falls and harm from falls in older people: best practice guidelines for Australian community care, 2009618 documents prepared by the Australian Commission on Safety and Quality in Health Care.
Screening
Falls risk screening provides an efficient means of identifying the individuals at greatest risk of falling who should have a comprehensive falls risk assessment performed. Falls risk screening generally involves reviewing only up to five brief items. A simple, easy-to-administer screen is to ask older people about their history of falls in the past 12 months and to assess their balance and mobility status. Those people with a history of one or more falls in the past year and who perform poorly in a simple test of gait or balance should be assessed further (Box 6).
Assessment
Assessment tools provide detailed information on the underlying deficits contributing to overall risk and should be linked to evidence-based tailored interventions. Assessing falls risk typically involves either the use of multifactorial assessment tools that cover a wide range of falls risk factors (Box 7), or individual functional mobility assessments, which focus on the physiological and functional domains of postural stability, including vision, strength, coordination, balance and gait (Box 8).
In order to develop an individualised care plan for preventing falls, the factors contributing to a person’s increased risk of falling need to be systematically and comprehensively identified. The risk factors presented in Box 8 have been identified as being more prevalent in people who fall compared with those who do not and should be assessed and managed if present.
Falls prevention strategies
There is now strong evidence from randomised controlled trials to support both single (Box 9) and multifactorial (Box 10) interventions in the prevention of falls in older people.
Exercise has been shown to be successful as a single intervention strategy in community-dwelling populations631 and is also effective in RACFs when part of multifactorial interventions.635 Exercise covers a wide range of physical tasks (balance, strength, flexibility, etc) delivered in numerous formats, some of which are likely to result in greater reductions in falls than others. Exercises should include balance training at a moderate to high intensity and should be ongoing.631 Overall, it is recommended that people aged between 60 and 80 years should be guided towards participating in tai chi group sessions with functional balance exercises if they have a low falls risk, and towards more targeted group-based exercise classes if they have a moderate falls risk. In people at high falls risk or who are older than 80 years, an individually tailored exercise program in the home, such as the Otago Exercise Programme, is likely to be most beneficial.
Multifactorial interventions involve identifying a range of risk factors associated with falls and offering interventions based on the identified risk profile (Box 10). Multifactorial interventions have been shown to be effective in a number of settings, and it is worth noting that in hospitals and RACFs, only multifactorial interventions have been shown to be effective in preventing falls.
1 Australian and New Zealand guidelines for recommended dietary intake (RDI) of calcium
|
Age (years)
|
RDI (mg/day)
|
|
|
Children
|
1–3
|
500
|
|
4–8
|
700
|
|
Girls
|
9–11
|
1000
|
|
12–13
|
1300
|
|
14–18
|
1300
|
|
Women
|
19–50
|
1000
|
|
51+
|
1300
|
|
Pregnancy
|
14–18
|
1300
|
|
19–50
|
1000
|
|
Boys
|
9–11
|
1000
|
|
12–13
|
1300
|
|
14–18
|
1300
|
|
Men
|
19–70
|
1000
|
|
71+
|
1300
|
|
| Source: National Health and Medical Research Council. |
2 Calcium content of key foods
|
Foods
|
Calcium content (mg per standard serve)
|
|
|
Milk, cheese and yoghurt
|
300–400
|
|
Tinned salmon and sardines
|
220–400
|
|
Calcium-set tofu
|
150
|
|
Nuts and tahini
|
65–110
|
|
Selected green vegetables
|
18–43
|
|
|
|
3 Recommended sun exposure requirements to meet adequate vitamin D levels42
| |
Summer
|
Winter
|
|
For people with moderately fair skin
|
|
How long?
|
6–7 minutes, most days
|
7–40 minutes (depending on latitude), most days
|
|
Body area exposed?
|
Arms exposed
|
As much bare skin exposed as practical
|
|
When?
|
At 10 am or 2 pm (standard time), 11 am or 3 pm (daylight saving time); avoid peak UV times
|
Midday
|
|
For people with darker skin*
|
|
How long?
|
18–42 minutes most days
|
21 minutes to 4 hours (depending on latitude)
|
|
Body area exposed?
|
Arms exposed
|
As much bare skin exposed as practical
|
|
When?
|
At 10 am or 2 pm (standard time), 11 am or 3 pm (daylight saving time); avoid peak UV times
|
Midday
|
|
|
UV = ultraviolet.
* Sun exposure requirements for people with dark skin are likely to be three to six times longer than for people with moderately fair skin.
|
4 The impact of selected exercises on bone health
|
Highly osteogenic
|
Moderately osteogenic
|
Low osteogenic*
|
Non-osteogenic*
|
|
|
Basketball/netball
|
Running/jogging
|
Leisure walking
|
Swimming
|
|
Impact aerobics
|
Brisk or hill walking
|
Lawn bowls
|
Cycling
|
|
Dancing/gymnastics
|
Resistance training
|
Yoga/Pilates/tai chi
|
|
|
Tennis
|
Stair climbing
|
|
|
|
Jump rope
|
|
|
|
|
|
*While certain exercises may have low or no osteogenic benefits, this should not be construed to imply that these exercises do not offer a wide range of other health benefits.
|
5 Recommendations for calcium intake by life stage (mg/day) — a comparison of current 2006 Australian National Health and Medical Research Council nutrient reference values4 and revised 2010 US Institute of Medicine dietary reference intakes99
| |
Calcium intake (mg/day)
|
|
Life stage
|
NHMRC4 EAR
|
IOM99 EAR
|
NHMRC4 RDI
|
IOM99 RDA
|
NHMRC4 UL
|
IOM99 UL
|
|
|
Infants
|
|
|
|
|
|
|
|
0–6 months
|
210 (AI)
|
200 (AI)
|
|
|
BM
|
1000
|
|
7–12 months
|
270 (AI)
|
260 (AI)
|
|
|
B/F
|
1500
|
|
Children
|
|
|
|
|
|
|
|
1–3 years
|
360
|
500
|
500
|
700
|
2500
|
2500
|
|
4–8 years
|
520
|
800
|
700
|
1000
|
2500
|
2500
|
|
Males
|
|
|
|
|
|
|
|
9–13 years
|
800–1050
|
1100
|
1000–1300
|
1300
|
2500
|
3000
|
|
14–18 years
|
1050
|
1100
|
1300
|
1300
|
2500
|
3000
|
|
19–30 years
|
840
|
800
|
1000
|
1000
|
2500
|
2500
|
|
31–50 years
|
840
|
800
|
1000
|
1000
|
2500
|
2500
|
|
51–70 years
|
840
|
800
|
1000
|
1000
|
2500
|
2500
|
|
> 70 years
|
1100
|
1000
|
1300
|
1200
|
2500
|
2000
|
|
Females
|
|
|
|
|
|
|
|
9–13 years
|
800–1050
|
1100
|
1000–1300
|
1300
|
2500
|
3000
|
|
14–18 years
|
1050
|
1100
|
1300
|
1300
|
2500
|
3000
|
|
19–30 years
|
840
|
800
|
1000
|
1000
|
2500
|
2500
|
|
31–50 years
|
840
|
800
|
1000
|
1000
|
2500
|
2500
|
|
51–70 years
|
1100
|
1000
|
1300
|
1200
|
2500
|
2000
|
|
> 70 years
|
1100
|
1000
|
1300
|
1200
|
2500
|
2000
|
|
Pregnancy
|
|
|
|
|
|
|
|
14–18 years
|
1050
|
1100
|
1300
|
1300
|
2500
|
3000
|
|
19–30 years
|
840
|
800
|
1000
|
1000
|
2500
|
2500
|
|
31–50 years
|
|
800
|
1000
|
1000
|
2500
|
2500
|
|
Lactation
|
|
|
|
|
|
|
|
14–18 years
|
1050
|
1100
|
1300
|
1300
|
2500
|
3000
|
|
19–30 years
|
840
|
800
|
1000
|
1000
|
2500
|
2500
|
|
31–50 years
|
840
|
800
|
1000
|
1000
|
2500
|
2500
|
|
| |
|
NHMRC recommendation lower than IOM recommendation.
|
| |
|
NHMRC recommendation higher than IOM recommendation.
|
|
|
AI = adequate intake. BM = breast milk. B/F = amount in breast milk and food. DRI = dietary reference intake. EAR = estimated average requirement. IOM = US Institute of Medicine. NHMRC = National Health and Medical Research Council. RDA = recommended dietary allowance. RDI = recommended dietary intake. UL = upper level of intake4 or upper level intake.99
|
6 Falls risk screening tools
|
Setting
|
Screening tool
|
|
|
Community
|
The timed “get up and go” test measures the time taken to rise from a chair, walk 3 m (with the patient’s usual assistive device), turn, return to the chair, and sit down. A time of 12 seconds or more indicates increased risk of falls.619
|
|
Hospital (subacute)
|
The St Thomas’s Hospital Risk Assessment Tool In Falling Elderly Inpatients (STRATIFY) contains five clinical fall risk factors. A positive score on ≥ 2 out of five items indicates increased risk of falls.620
|
|
Hospital (acute)
|
It has been suggested that clinical judgement to classify a patient as high risk for falls is equal to or even better than the use of screening tools.621
|
|
RACFs
|
Two different falls risk screening tools are required:622
- In people who can stand unaided, having poor balance or a positive score on two other risk factors (ie, previous falls, nursing home accommodation, or urinary incontinence) indicates an increased falls risk.
- In people who cannot stand unaided, having any one of three risk factors (previous falls, hostel residence, and using nine or more medications) increases the risk of falling twofold.
|
|
|
RACF = residential aged care facility.
|
7 Multifactorial falls risk assessment tools
|
Setting
|
Assessment tool
|
|
|
Community
|
QuickScreen Clinical Falls Risk Assessment is a risk assessment tool designed specifically for general practice and assesses previous falls, medication usage, vision, peripheral sensation, lower limb strength, balance and coordination.623
|
|
Hospital (subacute)
|
The Peninsula Health Falls Risk Assessment Tool (FRAT) has three sections: (1) falls risk status, (2) risk factor checklist, and (3) action plan.624
|
|
Hospital (acute)
|
Twelve components are included in the patient’s care plan dealing with both intrinsic risk factors and environmental risk factors.625
|
|
RACFs
|
Relatively few general falls risk assessment tools have been developed for use in RACFs, but the FRAT can also be used here.624
|
|
|
RACF = residential aged care facility.
|
8 Falls risk factors and validated assessment tools
|
Risk factor
|
Assessment tool
|
|
| Impairments in balance and gait |
Tinetti Performance-Oriented Mobility Assessment Tool626 |
| Cognitive impairment |
Mini-Mental State Examination627 |
| Incontinence |
Urinary and faecal assessment |
| Problems with feet and footwear |
Foot pain, safe-shoe checklist |
| Syncope/dizziness |
Tilt-Table Test628 |
| Medications |
Medication review |
| Poor vision |
Snellen eye chart |
| Environmental hazards |
Westmead Home Safety Assessment629 |
9 Successful single interventions in the community630
|
Risk factor
|
Single intervention
|
|
|
Impairments in balance and gait
|
Exercise*631
|
|
Problems with feet and footwear
|
Multifaceted podiatry intervention consisting of foot orthoses, advice on footwear, home-based foot and ankle exercises, and routine podiatry care632
|
|
Syncope/dizziness
|
Cardiac pacing in people with carotid sinus hypersensitivity and a history of syncope-related falls
|
|
Medications
|
Gradual and supervised withdrawal of psychoactive medications
Collaborative review and modification of medication by general practitioners and pharmacists
Vitamin D and calcium supplementation
|
|
Poor vision
|
Cataract surgery as soon as practicable for older people with visual impairment primarily related to cataracts
A home safety assessment and modification program designed to prevent falls for people with severe visual impairment
Provision of single-lens glasses should be considered for older people wearing multifocal glasses who take part in regular outside activities633
|
|
Environmental hazards
|
Occupational therapy interventions incorporating education and home hazard modification for high-risk older people634
|
|
|
* Exercise is described in detail in the text.
|
10 Successful multifactorial interventions in different settings630,635
|
Setting
|
Components of multifactorial intervention
|
|
|
Community
|
In older people at risk of falls, individualised assessment leading directly to tailored interventions is recommended
|
|
Hospital
|
Different combinations of supervised exercise and balance training, education, medication review, vitamin D with calcium supplementation, environmental review, walking aids and hip protectors have been successful at reducing falls in hospital
|
|
RACFs
|
Different combinations of supervised exercise and balance training, staff education, medication review, vitamin D with calcium supplementation, environmental adaptations, and hip protectors have been successful at reducing falls in RACFs
|
|
|
RACF = residential aged care facility.
|
White paper contributors*
Dr Christine Bailey, Postdoctoral Research Fellow, NorthWest Academic Centre, University of Melbourne, Melbourne, VIC
Professor Emily Banks, Professor of Epidemiology and Public Health, ANU College of Medicine, Biology and Environment, Australian National University, Canberra, ACT
Associate Professor Belinda Beck, Associate Professor, Musculoskeletal Research Group, Griffith Health Institute, School of Physiotherapy and Exercise Science, Griffith University, Gold Coast, QLD
Associate Professor Amanda Devine, Associate Professor School of Exercise, Biomedical and Health Science, Edith Cowan University, Perth, WA
Professor John Eisman, Senior Principal Research Fellow; Director, Bone Research Program, Garvan Institute of Medical Research; Professor of Medicine, University of New South Wales, Staff Endocrinologist, St Vincent’s Hospital, Sydney, NSW
Professor Dallas English, Professor and Director, Centre for Molecular, Environmental, Genetic and Analytic (MEGA) Epidemiology, Melbourne School of Population Health, University of Melbourne, Melbourne, VIC
Professor Maria A Fiatarone Singh, Professor, Exercise, Health and Rehabilitation Research Group, Faculty of Health Sciences; John Sutton Chair of Exercise and Sports Science; Professor, Sydney Medical School; Director, Exercise Division, Boden Institute of Obesity, Nutrition, Exercise and Eating, Sydney Medical School, University of Sydney, Sydney, NSW
Professor Graeme Jones, Professor of Rheumatology and Epidemiology, TW Senior Research Fellow, Menzies Research Institute, Hobart, TAS
Dr Jeffrey Lai, PhD Candidate, National Centre for Epidemiology and Population Health, Australian National University, Canberra; Medical Officer, The Canberra Hospital, Canberra, ACT
Associate Professor Robyn Lucas, Associate Professor, ANU College of Medicine, Biology and Environment, Australian National University, Canberra, ACT
|
Professor Rebecca Mason, Head, Physiology; Deputy Director Bosch Institute, University of Sydney, NSW
Professor Caryl Nowson, Chair of Nutrition and Ageing, Centre for Physical Activity and Nutrition Research (C-Pan), Deakin University, Melbourne, VIC
Dr Georgia Paxton, Paediatrician, Medical Coordinator Immigrant Health, Departments of General Medicine, Gastroenterology and Clinical Nutrition, Windmere Health Services Fellow, Royal Children’s Hospital, Melbourne, VIC
Associate Professor Nicholas Pocock, Senior Staff Specialist, Department of Nuclear Medicine, St Vincent’s Hospital, Sydney, NSW
Professor Richard Prince, Professor, School of Medicine and Pharmacology, Sir Charles Gairdner Hospital Unit, University of Western Australia, Perth, WA
Professor Ian Reid, Professor of Medicine and Endocrinology, Department of Medicine, University of Auckland, Auckland, New Zealand
Associate Professor Kerrie Sanders, Principal Research Fellow, NorthWest Academic Centre, University of Melbourne, Melbourne, VIC
Professor Robert Scragg, Professor of Epidemiology, Section of Epidemiology and Biostatistics, School of Population Health, University of Auckland, Auckland, New Zealand
Professor Markus Seibel, Professor of Medicine, University of Sydney; Director, Department of Endocrinology and Metabolism, Concord Hospital, Sydney, NSW
Professor Connie Weaver, Distinguished Professor and Head of Department, Nutrition Science, Purdue University, Indiana, USA
Dr Tania Winzenberg, Senior Research Fellow, Menzies Research Institute, Hobart, TAS
Dr Kun Zhu, Department of Endocrinology and Diabetes, Sir Charles Gairdner Hospital; School of Medicine and Pharmacology, University of Western Australia, Perth, WA
Paul Mitchell (editorial assistance), Director, Synthesis Medical NZ, Pukekohe, New Zealand
|
|
| * Positions at time of writing. |
Glossary
| Term |
Definition |
| Adequate intake (AI) |
Adequate intake is used when an estimated average requirement or recommended dietary intake cannot be developed; the average intake level is based on observed or experimentally determined approximations or estimates of nutrient intakes by a group (or groups) of apparently healthy people that are assumed to be adequate. |
| Bone mineral density (BMD) |
Bone density (or bone mineral density) is a medical term referring to the amount of matter per cubic centimetre of bones. BMD is used in clinical medicine as an indirect indicator of osteoporosis and fracture risk. This medical bone density is not a true physical “density”, which would be measured in mass per cubic volume. |
| Estimated average requirement (EAR) |
The estimated average requirement reflects the estimated median nutrient requirement and is particularly appropriate for applications related to planning and assessing intakes for groups of people. |
| Magnetic resonance imaging (MRI) |
Magnetic resonance imaging is a medical imaging technique used in radiology to visualise detailed internal structures. |
| Meta-analysis |
A meta-analysis combines the results of several studies that address a set of related research hypotheses. |
| Peripheral quantitative computed tomography (pQCT) |
Peripheral quantitative computed tomography is an imaging technique used for making measurements of the bone mineral density in a peripheral part of the body. It is useful for measuring bone strength. |
| Recommended dietary intake (RDI) |
The recommended dietary intake is derived from the estimated average requirement and meets or exceeds the nutrient requirements for 97.5% of the population. The analogous term often used in the US is “recommended dietary allowance (RDA)”. |
| Systematic review |
A systematic review is a literature review focused on a research question that tries to identify, appraise, select and synthesise all the high quality research evidence relevant to that question. |
| Upper level of intake (UL) |
As intake increases above the upper level of intake, the potential risk of adverse effects may increase. The upper level of intake is the highest average daily intake that is likely to pose no risk of adverse effects to almost all individuals in the general population. In the US, the abbreviation UL refers to “tolerable upper intake level”. |

 more_vert
more_vert