An effective system of stewardship is needed to optimise the use of pathology tests
Many hospital clinical pathology laboratories presently experience annual increases in workload of 5%–10%.1 Such increases in demand are often not accompanied by concomitant increases in laboratory resources. This environment presents a significant challenge to laboratories that have no control over test-ordering patterns. Compounding this situation is the fact that many pathology tests are inappropriate or unnecessary, as they have no impact on patient care. The extent of inappropriate pathology test ordering in Australia is unknown, but a United Kingdom report on National Health Service pathology services estimated that 25% of all requests were unnecessary or inappropriate.2 Such tests are ordered for a variety of reasons, often in the belief that more testing equates to better patient care. Unfortunately, this is not always the case, and in some circumstances the opposite may be true.
Ozbug3 is a well established closed and moderated email list largely but not solely restricted to members of the Australasian Society for Infectious Diseases, predominantly comprising Australian and New Zealand infectious diseases physicians but also including registrars, medical microbiologists and infection prevention practitioners. There are about 800 subscribers, who discuss a broad range of topics. I asked the following question on Ozbug: “What microbiology laboratory investigation would you consider to be the one, although requested, results in least patient benefit? or What do you consider to be the most useless of microbiology tests?” The unexpectedly large number of responses (140) to this question and the ensuing rich discussion are the stimuli for this article.
My aim here is to discuss and attempt to understand inappropriate or unnecessary pathology testing, to define the drivers for and impact of such testing, and to suggest interventions to improve the use of pathology services. I will focus on hospital pathology services and provide specific examples from my discipline of microbiology.
Inappropriate pathology test ordering
Tests that are ordered but the results of which are never viewed by the clinician are of no use to the management of the specific patient. Duplicate tests or tests performed before initial testing results are available are unnecessary. Similarly useless tests include those that, no matter what the result, will not impact on patient care. Some serological diagnoses require collection of initial and convalescent sera. In many such circumstances, only a single sample is obtained and this is of no use. Many of the serological tests undertaken for the investigation of fatigue have a low likelihood of a useful result and may give the patient false hope of a result that will lead to a definitive diagnosis and effective therapeutic intervention. A serology test should only be performed when the clinical illness and epidemiology support that diagnosis. Otherwise a false-positive result may complicate patient management. These last two points are exemplified by Lyme disease serology, which is often performed in a setting of vague, non-specific symptoms in a patient who has never visited a known endemic region or country.
For bacterial culture, a dry swab in a specimen jar is unlikely to be useful. A midstream urine specimen that has a normal urinalysis result is most unlikely to identify a pathogen. A recent trend is to swab environmental services or inanimate objects for resistant organisms. This often occurs in the absence of epidemiological evidence to support such a link, and such swabbing should be resisted.
Generally, microbiology tests for clearance, such as repeat throat and nose swabs for respiratory viruses and repeat stool tests for Clostridium difficile, are unnecessary or not recommended.
Other common individual tests suggested by Ozbug correspondents as inappropriate or unnecessary are included in Box 1. Ozbug correspondents acknowledge that for many of the tests mentioned, it is not the test itself that is under scrutiny but the use of that test, and also that these tests may be useful in specific circumstances or jurisdictions. Laboratories also have the responsibility to offer tests that have been validated for the purpose for which they are offered.
Factors contributing to inappropriate pathology ordering
The prime reason for ordering pathology testing is to optimise patient diagnosis and management. Most practitioners agree on the importance of prudent use of pathology services. However, there may be other less apparent drivers for suboptimal pathology test ordering. Such testing may be tied to the patient’s or the family’s expectations rather than to an actual need for such testing. The physician’s anxiety or fear of missing a diagnosis may generate the feeling that something needs to be done, leading to overinvestigation without a clear rationale for that testing. Junior doctors may order according to peer perception or because they are concerned that their consultant may criticise them if the test has not been requested. In some circumstances when a doctor is time pressured, ordering pathology tests may be an easier course than the timely consideration of management options.
Other less than ideal reasons to order pathology tests include: “wouldn’t it be nice to know”, “I cannot find (or have not looked for) the previous result”, “I may want to publish the case in the future” and “I do not believe the result from the first laboratory and I want to send it to a second laboratory”. Some individual factors contributing to suboptimal testing, suggested by Ozbug correspondents, are summarised in Box 2.
Pathology laboratories may also contribute to the number of inappropriate tests. New technology with new testing menus may be introduced before there is evidence that such developments have a favourable impact on patient outcomes.4
Risks of inappropriate pathology ordering
Some tests are not only unnecessary but may be misleading or even harmful. The receipt and subsequent processing of saliva when sputum is ordered may identify transient oral colonising bacteria such as Streptococcus pneumoniae or methicillin-resistant Staphylococcus aureus. This may do a patient harm if the organism is then assumed to be the aetiological cause of the pneumonia, targeted treatment is given and the real cause of pneumonia is overlooked.
When inappropriate or unnecessary tests are ordered, there is a risk of a false-positive result, leading to further unnecessary testing, other investigations and even unnecessary treatments with attendant adverse effects. Ober has described this cascade effect, highlighting that a “normal range” typically includes 95% of all normal subjects, with up to 5% of normal subjects given an abnormal result.5 With modern multichannel analysers, more often used in other pathology disciplines, the chance of a false-positive result is further increased.
Inappropriate pathology testing consumes laboratory resources, both budgetary and labour. This may, especially in more manual disciplines such as microbiology, lead to delays in processing and increase the turnaround time for specimens from the patients in greatest need.
Strategies to improve pathology test ordering
There has been much discussion among the Ozbug group concerning possible strategies to improve microbiology test ordering. Individual strategies suggested by Ozbug correspondents are shown in Box 3. There are a limited number of studies documenting the impact of a strategy targeting a specific test with a decrease in the ordering of that test during the period of observation.6 However, such interventions generally do not tackle the breadth of pathology testing, and the long-term sustainability of such interventions is questionable.
Overall, the Ozbug discussions emphasise the need for an ongoing system of stewardship to ensure the optimal use of pathology resources. To be effective, a system needs to be developed together with all the major stakeholders, have a strong and iterative educational component, be evidence-based, include a system of regular audit with feedback, and especially target those tests that are high cost, resource expensive and frequently used inappropriately. Orders from clinicians should be considered requests for testing as well as for specialist pathologist input. Within my own discipline, the clinical microbiologist should take an active lead in decisions about testing menus and indications, specimen acceptability and acceptance, testing quality, and test interpretation.
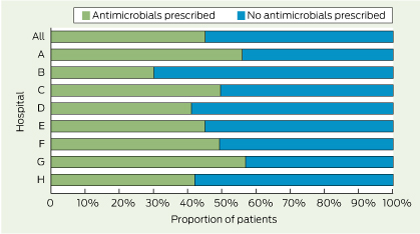
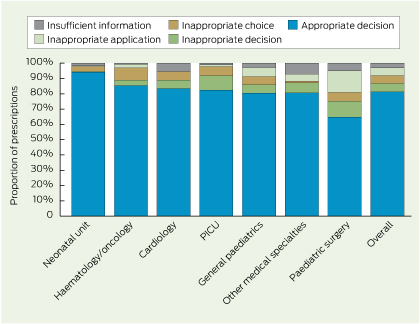
Just as antimicrobial stewardship has now become a national standard for hospital accreditation, a system of pathology stewardship would optimise the use of pathology resources. This is not a new concept. In 1922, Peabody wrote:
Good medicine does not consist in the indiscriminate application of laboratory examinations to a patient, but rather in having so clear a comprehension of the probabilities and possibilities of a case as to know what tests may be expected to give information of value.7
1 Ozbug correspondents’ examples of inappropriate microbiology test ordering*
- Most extra tests performed on cerebrospinal fluid when no abnormalities were found on microscopy
- Routine cultures of vascular catheters
- Vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus surveillance cultures in unquarantined patients
- Parasites in stools in hospitalised patients
- Surveillance blood cultures in asymptomatic patients
- Streptococcal, herpes, typhoid fever (eg, Widal test) and Lyme disease serology
- Legionella and pneumococcal urinary antigens in patients with normal chest x-ray results
- Repeated bacterial surveillance cultures of endoscopy equipment
* Note: In some specific circumstances these tests may be appropriate.
2 Ozbug correspondents’ reports of potential factors contributing to inappropriate test ordering
- Suboptimal teaching of undergraduates and graduates
- Pressure of work for both clinicians and pathologists
- Lack of pathologist input for test menu development and specimen suitability information
- Clinicians’ poor understanding of test reliability and validity
- Clinicians’ lack of knowledge and concern about pathology costs
- Ease of ordering tests electronically or using prestamped request slips
- Income generation of some pathology testing
- Acceptance of public pathology as a learning environment that encourages more pathology
- Fear of litigation
3 Strategies suggested by Ozbug correspondents to improve microbiology test ordering
- Enhanced education of medical students and graduates
- Pathology ordering audit and feedback
- Increased collaboration and engagement with clinicians
- Development of rejection rules such as minimum retest intervals
- Display of costs of pathology tests with pathology results
- Standardisation of investigations for specific clinical syndromes
- Development and promulgation of golden rules regarding pathology testing
- Pathology rotations for junior medical staff
- Prevention of duplicate testing

 more_vert
more_vert