The health of Australia’s prisoners 2015 is the 4th report produced by the Australian Institute of Health and Welfare on the health and wellbeing of prisoners. The report explores the conditions and diseases experienced by prisoners; compares, where possible, the health of prisoners to the general Australian community and provides valuable insight into the use of prison health services. New to the 2015 report are data on the disabilities or long-term health conditions of prisoners entering the prison system (prison entrants), self-assessed mental and physical health status of prisoners and data on smoke-free prisons.
Preference: Infectious Diseases and Parasitology
434
Cardiovascular disease, diabetes and chronic kidney disease—Australian facts: Aboriginal and Torres Strait Islander people
Cardiovascular disease, diabetes and chronic kidney disease—Australian facts is a series of 5 reports by the National Centre for Monitoring Vascular Diseases at the Australian Institute of Health and Welfare that describe the combined burden of cardiovascular disease (CVD), diabetes and chronic kidney disease (CKD).This report on Aboriginal and Torres Strait Islander people presents up-to-date statistics on risk factors, prevalence, hospitalisation and deaths from these 3 chronic diseases. It examines age and sex characteristics and variations by geographical location and compares these with the non-Indigenous population.
[Articles] Effects of decreases of animal pollinators on human nutrition and global health: a modelling analysis
Declines in animal pollinators could cause significant global health burdens from both non-communicable diseases and micronutrient deficiencies.
The epidemiology of tuberculosis in children in Australia, 2003–2012
Tuberculosis (TB) in children has received increasing attention during the past decade, and the World Health Organization first estimated the global burden in 2012. In 2014, it announced that there had been 550 000 cases of TB in children aged 0–14 years in 2013,1 but acknowledged that limitations to case detection meant that this was likely to be an underestimate.2 While children with TB are not a major source for disease transmission, TB is an important cause of childhood morbidity and mortality in settings where TB is endemic; further, an incident TB case in a young child is an important sentinel indicator of recent transmission in their community.3,4 The strategic plan for TB control in Australia recognises the incidence of TB in Australian-born children as a useful indicator for enhanced surveillance in high-risk groups and for monitoring progress in eliminating TB in the Australian-born population.5,6
The incidence of TB in Australia has been low and stable for decades, with reported rates of 6.8 and 5.8 cases per 100 000 population for 1990 and 2012 respectively.6 Recent immigrants from TB-endemic countries account for most cases now detected.7 Investigations of children from immigrant families have found a high prevalence of infection with Mycobacterium tuberculosis.8 As a detailed analysis of the epidemiological characteristics of the national burden of TB in children in Australia has not been published, we aimed to determine the incidence and epidemiology of TB in this population over the past decade.
Materials and methods
The notification of TB is mandatory in Australia, and all cases are reported to the National Notifiable Diseases Surveillance System (NNDSS), which coordinates national disease surveillance under the auspices of Communicable Diseases Network Australia (CDNA).5 All medical professionals, laboratories and other health practitioners are required by state and territory public health legislation to notify cases of TB to the relevant health departments. Under the National Health Security Act 2007, which permits the exchange of health information between federal and state and territory health departments, de-identified data for TB cases that fulfil the national case definition are transferred to the NNDSS.9
Data for all children aged 0–14 years diagnosed with TB and reported to the NNDSS between 1 January 2003 and 31 December 2012 were included in our analysis. De-identified data for age, sex, Indigenous status, country of birth, year of arrival, residency status, site of disease, case classification, method of case identification, risk factors, laboratory results and treatment outcomes were provided. The definitions used by CDNA for TB case classification and treatment outcomes, as well as for residency and immigration status, are listed in Box 1.
Analysis was performed with Stata 9.0 (StataCorp). Proportions of categorical variables were compared using the Fisher exact test. Incidence rates and 95% confidence intervals were calculated. Notification rates were calculated using estimated resident population data from the Australian Bureau of Statistics,10,11 including population data for the Indigenous Australian population.12,13 National TB case load and rates were obtained from the online NNDSS application for generating summary data.14
Ethics approval
Approval for the investigation was granted by CDNA, and ethics approval was granted by the Australian Capital Territory Health Human Research Ethics Committee (reference ETH.1.14.025).
Results
A total of 538 cases of TB in children were notified in Australia between 2003 and 2012, representing 4.6% of the total case load for that period (Box 2). The average annual notification rate for children was 1.31 cases per 100 000 child population, ranging from 0.92 in 2003 to 1.65 per 100 000 children in 2006. These rates should be compared with overall case notification rates of 5.0 per 100 000 and 5.9 per 100 000 total population in 2003 and 2006 respectively.14 Case classification was reported for 97.4% (524/538) of child notifications, and 97.7% of these (512/524) were new cases. Of the remaining 12 cases, seven relapsed after full treatment in Australia, two after partial treatment in Australia, and three after treatment overseas. The greatest number of notified cases were from Victoria, and the lowest from Tasmania; the notification rate was highest in the Northern Territory.
Distribution by age, sex and site of disease
The age of the patient was reported in all notified cases. The average annual notification rates were significantly higher for those aged 0–4 years than for those aged 5–9 years (P = 0.0004) or 10–14 years (P = 0.03). Just over half the patients (51.7%, 278/538) were girls. The anatomical site of disease was recorded for 97.2% (523/538) of notified cases; pulmonary disease was the most frequently reported site in all age bands. Eight of the 13 patients with TB meningitis were less than 5 years old (Box 3).
Tuberculosis notifications by population subgroup
Box 4 and Box 5 list the case numbers and annual case notification rates by place of birth. A country of birth was reported for 97.4% (524/538) of patients; of these, 44% (230/524) were born in Australia, including 37 Indigenous children (of whom 20 were notified in the NT). The annual notification rate among Australian-born children was higher for Indigenous than for non-Indigenous children (1.70 [95% CI, 1.20–2.34] v 0.56 [95% CI, 0.48–0.65] per 100 000 population).
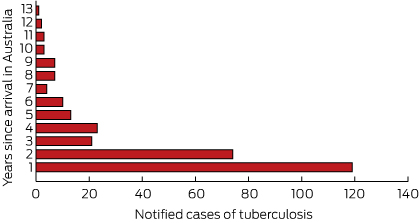
Annual notification rates were significantly higher for overseas-born children than for Australian-born children (Box 4). Of the 294 notifications for children reported as born overseas, the highest number came from Papua New Guinea (64 patients) and the Sudan (59 patients) (Box 5). Data on the year of arrival were available for 97.6% of the patients reported as born overseas (287/294); 40.4% (119/294) of these cases were notified within a year of their arrival in Australia, 65.6% within 2 years, and 85.0% within 5 years (Box 6). Residency status was available for 77.8% (229/294) of the overseas-born children: 36% (83/229) had refugee or humanitarian status, 35% (81/229) were permanent residents, and 20% (46/229) were children diagnosed while accessing health care treatment in the Torres Strait Treaty Zone. Eight of the remaining 19 children were visitors, four were unauthorised arrivals, one was an overseas student, and six were classified as “other” (with no further information).
Case detection and prevalence of known risk factors
Data on the method of case detection were available for 79.4% (427/538) of children. Most cases (54.3%, 232/427) were diagnosed by passive case detection after clinical presentation. Active screening identified further cases: 37.0% of cases (158/427) were detected by contact screening and 8.7% (37/427) by onshore post-arrival immigration screening. Contact with an infected person was reported for 34% (78/232) of patients diagnosed after clinical presentation, and for 22% (8/37) of those detected by immigration screening. Although 158 cases were recorded as having been detected by contact screening, a contact was not identified in 19% of these cases (30/158). There were 111 cases with no recorded information about the method of detection, and 44% (49/111) of these were reported to have had contact with an infected person.
Overall, a household or other close contact with TB was identified in 263 cases (48.8%), and 226 (42.0%) reported at least 3 months’ cumulative travel to or residence in at least one high-risk country that was not their country of birth (Box 4). Sixty-eight children had both of these risk factors for TB. Five children were reported to be infected with HIV, but data on the number of patients who had been tested for HIV were not available.
Culture and drug susceptibility
A sputum culture was known to have been prepared for 25.1% (135/538) of children with TB, 59% (79/135) of which were positive for M. tuberculosis; this represented 15% of all notified cases (Box 4). The yield from sputum culture was similar for Australian-born and overseas-born patients. The criteria that determined whether sputum was collected from children with suspected TB were not recorded. A sputum culture result was recorded for 34.2% of children with pulmonary (with or without extrapulmonary) disease (119/348), of which 66% (78/119) were culture-positive. Of the 175 children classified as having only extrapulmonary disease, sputum culture results were available for 9% (16/175), of which one was culture-positive.
Results of susceptibility testing were available for 29.4% (158/538) of notified cases; 78.5% of tested isolates (124/158) were fully susceptible to first-line anti-TB drugs. Eight children presented with multidrug-resistant TB (MDR-TB), of whom six were under 4 years of age; seven of these children were from Papua New Guinea, one from Ethiopia. The prevalence of MDR-TB among all children with TB was thus 1% (8/538), or 5% (8/158) among those for whom susceptibility test results were available. Of the 46 children treated under the Torres Strait Treaty, 40 were culture-positive: 12% (5/40) of these children had monoresistant TB, 17% (7/40) MDR-TB, and 47% (19/40) fully susceptible TB; there were no susceptibility data for the remaining nine cases.
Treatment outcomes
Treatment outcome was classified as either “cured” or “successfully completed treatment” for 89.4% (481/538) of children; seven were still undergoing treatment, while 23 had transferred out of Australia or were not followed up at the end of the data collection period (Box 4). Five children died of TB, one an Australian-born Indigenous child with fully drug-sensitive TB. The other four deaths were of children from Papua New Guinea with smear-positive pulmonary TB, three of whom had additional sites of disease (meningeal, peritoneal and disseminated TB). One was a relapsed case of MDR-TB, while the others were new cases with fully drug-susceptible TB. Of the 46 children with TB treated under the Torres Strait Treaty, 27 (59%) were cured or had completed treatment, 13 (28%) returned to Papua New Guinea with an unknown outcome, and three (7%) had died; one defaulted from treatment, one was not followed up, and one was still being treated.
Discussion
This study provides original, comprehensive and recent data on the epidemiological and clinical characteristics of TB in children in Australia. Paediatric cases of TB accounted for 5% of the total national TB case load, a finding similar to those of recent reports from other low-incidence settings.15–17 National rates of TB in children in Australia have not changed markedly over the past 10 years, consistent with the stable rate for the overall population.18
Our findings suggest that recent immigration has had a greater influence on the burden of TB in children than transmission within Australia. In most cases (56%), the reported country of birth was outside Australia, and this proportion was higher than the corresponding figures for New Zealand, the United States and western Europe, where 26%–31% of patients with TB were born outside the respective country.16,17,19 The notification rate of 9.57 per 100 000 overseas-born children per year is lower than the rate of 35–37 per 100 000 overseas-born children reported for the United Kingdom (1999–2006) and the Netherlands (1993–2012).15,20 This difference may reflect the fact that there was no pre-migration screening in those countries during the reported periods, so that all cases were detected and notified after arrival.
Despite the strong link with recent immigration that we found, only a minority of children with TB (8.7%) were detected by specific onshore post-arrival immigration screening. Passive case detection identified most cases, including the overseas-born children who were typically diagnosed within 2 years of their arrival. The frequency of post-arrival case detection may indicate that the offshore screening process is inadequate,21 or that most overseas-born children with TB did not have active disease at the time of their initial screening. This suggests that there is potentially a role for preventive therapy in immigrant children from high-incidence countries with latent TB infection (LTBI), which could be supported by introducing improved pre-migration screening of younger children. A chest x-ray is performed on all applicants for a permanent or provisional visa for Australia aged 11 years and over; in younger applicants, a chest x-ray is performed only when there is a history of contact with TB or if clinically indicated by symptom screening.22 The case detection rate by immigration screening in Australia is higher in adult than in child immigrants.23 The lower detection rate for children probably reflects the epidemiology of TB, although it may also indicate an inadequate pre-migration screening process that is based largely on physical examination. The US has recently enhanced pre-migration screening of children aged 2–14 years in countries where the WHO estimates that TB incidence is at least 20 cases per 100 000 persons by including screening for LTBI; this involves a tuberculin skin test or interferon-γ release assay, which, if positive, is followed by a chest x-ray.24
Current opportunities for prevention that are supported by our findings include contact investigations. While BCG status was not reported for the cases we analysed, BCG vaccination of young children before they travel for extended periods to high-incidence countries could play a greater role.15,19,25 Opportunities for prevention in at-risk children are often missed.15
The higher notification rate for Indigenous than for non-Indigenous Australian-born children is similar to the pattern reported for the general population.6,26 In New Zealand, higher rates of TB were found in children of Pacific Island and Maori background than for other locally born children.19 An active program for diagnosing and treating LTBI in Indigenous people by contact, community, school and prison screening is one strategy used in some areas to better prevent and to ultimately eliminate TB.27 The complex nature of TB in Indigenous Australians has been discussed elsewhere.28
A large number of TB notifications were of children with refugee or humanitarian status or who used Australian medical care under the Torres Strait Treaty. It is recommended that all refugees, including children, be screened for LTBI and then referred to local TB services if a positive culture is obtained.29 This provides an opportunity for treating LTBI in refugee groups. The access of Papua New Guinea nationals, including children, to medical services in the Torres Strait Treaty Zone has recently become a major political and clinical management problem because of the high prevalence of MDR-TB in this population.30
The strengths of our study include the fact that we ascertained cases from a national notifiable disease register containing data from all Australian jurisdictions. There are, however, potential limitations. Although we did not formally validate the recorded data, discrepancies were noted, suggesting that incorrect information may have been entered. Data were incomplete for some important variables, including the method of case detection and whether HIV testing was undertaken. The case detection method was unknown for 20% of cases, but recognition of TB in children is an important sentinel indicator of recent transmission.4 Further, it is not known whether the data on specimen submission for laboratory-based diagnosis were complete. Laboratory confirmation and drug susceptibility testing are important indicators of diagnostic accuracy, and epidemiological trends, including those for MDR-TB, also have implications for clinical management. Finally, while all Australian jurisdictions were included in our study, it has been shown in the UK that TB in children can be both under- and over-reported.31 This facet of the problem has not been assessed in Australia.
In conclusion, there is a low burden of paediatric TB in Australia, but the rate has not changed over the past decade. The highest rates are among children born overseas, emphasising the potential value of broadening pre-migration screening to further reduce the burden of TB in children in Australia.
Box 1 –
Definitions used by Communicable Diseases Network Australia for tuberculosis (TB) case classification, and for residency and immigration status
Case classification
- New case: A patient who has never been treated for TB, or who was treated for less than a month
- Relapse following treatment in Australia: A patient previously treated for TB in Australia, declared cured or treatment completed, who is subsequently diagnosed with TB
- Relapse following treatment overseas: A patient previously treated for TB overseas, declared cured or treatment completed, who is subsequently diagnosed with TB
- TB following partial treatment: A patient who is deemed to have completed a partial course of treatment, in Australia or overseas, who is subsequently diagnosed with TB
Australian residency/immigration status
- Refugee/humanitarian: A person in humanitarian need overseas, or a person already in Australia who arrived on a temporary visa or in an unauthorised manner, and is claiming asylum
- Permanent resident: A person who holds a permanent visa and is usually resident in Australia
- Visitor: A person entering Australia temporarily for tourism, to visit family and friends, to undergo prearranged medical treatment, or for business-related purposes
- Overseas student: A person studying or seeking study, training or skills development in Australia
- Unauthorised person: A non-citizen unlawfully present in Australia
- Residents of Papua New Guinea covered by the Torres Strait Treaty who have accessed TB treatment with Queensland Health
Box 2 –
Notified cases of tuberculosis (TB) in children (aged 0–14 years), and case notification rates in Australia, 2003–2012
|
|
NSW/ACT |
NT |
Qld |
SA |
Tas |
Vic |
WA |
Australia* |
National TB case load (child cases as percentage) |
||||||
|
|
|||||||||||||||
|
2003 |
15 |
4 |
4 |
2 |
0 |
11 |
1 |
37 (0.92; 0.65–1.27) |
985 (3.8%) |
||||||
|
2004 |
10 |
0 |
13 |
0 |
0 |
18 |
6 |
47 (1.17; 0.86–1.56) |
1062 (4.4%) |
||||||
|
2005 |
17 |
1 |
5 |
1 |
1 |
31 |
7 |
63 (1.57; 1.21–2.01) |
1086 (5.8%) |
||||||
|
2006 |
18 |
2 |
12 |
0 |
0 |
29 |
5 |
66 (1.65; 1.28–2.10) |
1220 (5.4%) |
||||||
|
2007 |
21 |
8 |
5 |
2 |
0 |
13 |
5 |
54 (1.35; 1.01–1.76) |
1131 (4.8%) |
||||||
|
2008 |
16 |
3 |
7 |
1 |
1 |
25 |
3 |
56 (1.40; 1.06–1.82) |
1217 (4.7%) |
||||||
|
2009 |
16 |
0 |
7 |
2 |
0 |
23 |
7 |
55 (1.37; 1.04–1.79) |
1305 (4.2%) |
||||||
|
2010 |
16 |
5 |
10 |
3 |
0 |
7 |
8 |
49 (1.22; 0.91–1.62) |
1365 (3.6%) |
||||||
|
2011 |
13 |
1 |
17 |
4 |
0 |
18 |
8 |
61 (1.52; 1.17–1.96) |
1384 (4.4%) |
||||||
|
2012 |
15 |
2 |
10 |
3 |
0 |
15 |
5 |
50 (1.25; 0.91–1.65) |
1317 (3.8%) |
||||||
|
|
|||||||||||||||
|
Total |
157 |
26 |
90 |
18 |
2 |
190 |
55 |
538 (1.31; 1.20–1.43) |
12 074 (4.6%) |
||||||
|
Five-year case notification rates (per 100 000 children per year, with 95% CI), 2003–2007 |
|||||||||||||||
|
|
NSW/ACT |
NT |
Qld |
SA |
Tas |
Vic |
WA |
Australia |
|||||||
|
|
|||||||||||||||
|
Cases |
81 |
15 |
39 |
5 |
1 |
102 |
24 |
267 |
|||||||
|
Rate |
1.17 (0.93–1.45) |
4.71 (2.64–7.77) |
0.97 (0.69–1.32) |
0.35 (0.14–0.82) |
0.21 (0.01–1.15) |
2.12 (1.73–2.58) |
1.18 (0.76–1.76) |
1.37 (1.21–1.54) |
|||||||
|
Five-year case notification rates (per 100 000 children per year, with 95% CI), 2008–2012 |
|||||||||||||||
|
|
NSW/ACT |
NT |
Qld |
SA |
Tas |
Vic |
WA |
Australia |
|||||||
|
|
|||||||||||||||
|
Cases |
76 |
11 |
51 |
13 |
1 |
88 |
31 |
271 |
|||||||
|
Rate |
1.07 (0.84–1.33) |
3.32 (1.66–5.94) |
1.16 (0.86–1.52) |
0.90 (0.47–1.53) |
0.21 (0.01- 1.16) |
1.75 (1.40–2.16) |
1.39 (0.95–1.97) |
1.32 (1.16–1.48) |
|||||||
|
|
|||||||||||||||
|
* In parentheses: rates per 100 000 per year (95% CI). |
|||||||||||||||
Box 3 –
Sites of disease, notified cases and rates of tuberculosis (TB) by 5-year age bands
|
|
Age group |
||||||||||||||
|
0–4 years |
5–9 years |
10–14 years |
Total |
||||||||||||
|
|
|||||||||||||||
|
Rate per 100 000 children (95% CI) |
1.58 (1.38–1.81) |
1.08 (0.92–1.27) |
1.27 (1.09–1.47) |
1.31 (1.20–1.43) |
|||||||||||
|
Sites of disease (number, as percentage of age group) |
|||||||||||||||
|
Pulmonary only |
121 (56%) |
68 (47%) |
83 (47%) |
272 (50.6%) |
|||||||||||
|
Pulmonary plus other sites |
25 (12%) |
29 (20%) |
22 (13%) |
76 (14.1%) |
|||||||||||
|
Extrapulmonary only |
67 (31%) |
43 (29%) |
65 (37%) |
175 (32.5%) |
|||||||||||
|
Lymph node |
|
|
|
113 (21.0%) |
|||||||||||
|
Disseminated TB |
|
|
|
19 (3.5%) |
|||||||||||
|
Pleural |
|
|
|
32 (5.9%) |
|||||||||||
|
Bone/joint |
|
|
|
22 (4.1%) |
|||||||||||
|
Meningeal |
|
|
|
13 (2.4%) |
|||||||||||
|
Peritoneal |
|
|
|
7 (1.3%) |
|||||||||||
|
Genitourinary |
|
|
|
5 (0.9%) |
|||||||||||
|
Not recorded |
3 (1%) |
6 (4%) |
6 (3%) |
15 (2.8%) |
|||||||||||
|
|
|||||||||||||||
|
Total |
216 (40.1%) |
146 (27.1%) |
176 (32.7%) |
538 |
|||||||||||
Box 4 –
Selected characteristics of Australian-born and overseas-born children with tuberculosis
|
|
Born in Australia |
Born overseas |
Birthplace unknown |
Total |
|||||||||||
|
Indigenous |
Non-Indigenous |
Total |
|||||||||||||
|
|
|||||||||||||||
|
State or territory* |
|
|
|
|
|
|
|||||||||
|
NSW/ACT |
6 |
76 |
82 (53%) |
73 (47%) |
2 |
157 |
|||||||||
|
NT |
20 |
1 |
21 (81%) |
5 (19%) |
0 |
26 |
|||||||||
|
Qld |
8 |
6 |
14 (16%) |
75 (84%) |
1 |
90 |
|||||||||
|
SA |
3 |
8 |
11 (61%) |
7 (39%) |
0 |
18 |
|||||||||
|
Tas |
0 |
1 |
1 (50%) |
1 (50%) |
0 |
2 |
|||||||||
|
Vic |
0 |
90 |
90 (50%) |
100 (50%) |
11 |
190 |
|||||||||
|
WA |
0 |
11 |
11 (20%) |
44 (80%) |
0 |
55 |
|||||||||
|
Australia |
37 |
193 |
230 (43.9%) |
294 (56.1%) |
14 |
538 |
|||||||||
|
Annual case notification rates, per 100 000 children (95% CI) |
1.70 (1.20–2.34) |
0.56 (0.48–0.65) |
0.61 (0.53–0.69) |
9.57 (8.51–10.73) |
|
1.31 (1.20–1.43) |
|||||||||
|
Presentation |
|
|
|
|
|
|
|||||||||
|
Clinical presentation |
11 (30%) |
85 (44%) |
96 (43%) |
129 (57%) |
7 |
232 (43.1%) |
|||||||||
|
Contact tracing |
16 (43%) |
91 (47%) |
107 (70%) |
47 (31%) |
4 |
158 (29.4%) |
|||||||||
|
Screening |
0 |
1 |
1 |
36 (97%) |
0 |
37 (6.9%) |
|||||||||
|
No data |
10 (27%) |
16 (8%) |
26 (24%) |
82 (76%) |
3 |
111 (20.6%) |
|||||||||
|
Risk factors |
|
|
|
|
|
|
|||||||||
|
Household contact |
29 (78%) |
120 (62%) |
149 (57%) |
114 (43%) |
0 |
263 (48.9%) |
|||||||||
|
Past travel to or through, or residence in a high-risk country or countries† |
3 (8%) |
32 (17%) |
35 (15%) |
190 (84%) |
1 |
226 (42.0%) |
|||||||||
|
Microbiology results |
|
|
|
|
|
|
|||||||||
|
Positive sputum culture (number, percentage of cultures) |
7/10 |
14/27 |
21/37 |
57/97 |
1/1 |
79/135 [59%] |
|||||||||
|
Any culture-positive results |
26 (70%) |
114 (59%) |
140 (41%) |
204 (59%) |
1 |
345 (64.1%) |
|||||||||
|
Treatment outcome‡ |
|
|
|
|
|
|
|||||||||
|
Completed treatment or cured |
34 (92%) |
186 (96%) |
220 (96%) |
260 (88%) |
1 |
481 (89.4%) |
|||||||||
|
Defaulted from treatment |
0 |
2 |
2 |
2 |
0 |
4 |
|||||||||
|
Died of tuberculosis |
1 |
0 |
1 |
4 |
0 |
5 |
|||||||||
|
Died of other cause |
0 |
1 |
1 |
1 |
0 |
2 |
|||||||||
|
Interrupted treatment |
1 |
0 |
1 |
0 |
0 |
1 |
|||||||||
|
Still under treatment |
1 |
0 |
1 |
6 |
0 |
7 |
|||||||||
|
Transferred out of Australia or not followed up |
0 |
4 |
4 |
19 |
0 |
23 |
|||||||||
|
Treatment failure |
0 |
0 |
0 |
0 |
1 |
1 |
|||||||||
|
|
|||||||||||||||
|
Except for state or territory data, percentages are column percentages; percentages < 5% are not given. * The denominator is the number of cases with known place of birth. † For at least 3 months (cumulative) at any time during the patient’s life, and where the country is not their country of birth. ‡ No data on outcome available for 14 cases. |
|||||||||||||||
Box 5 –
Notified cases and case notification rates of tuberculosis for frequently reported countries of birth, by residency status
|
Country of birth |
Cases 2003–2007 |
Rate 2003–2007 |
Cases 2008–2012 |
Rate 2008–2012 |
Total cases |
Average case notification rate* |
|||||||||
|
|
|||||||||||||||
|
Australia |
103 |
0.55 (0.45–0.67) |
127 |
0.66 (0.55–0.79) |
230 |
0.61 (0.53–0.69) |
|||||||||
|
Papua New Guinea |
23 |
262.56 (166.44–393.96) |
41 |
377.18 (270.67–511.69) |
64 |
326.03 (251.08–416.34) |
|||||||||
|
Somalia |
8 |
249.22 (107.60–491.07) |
6 |
276.5 0 (101.47–601.82) |
14 |
260.22 (142.27. 436.61) |
|||||||||
|
Ethiopia |
7 |
154.87 (62.26–391.09) |
4 |
65.04 (17.72–166.53) |
11 |
103.1 (51.46–184.45) |
|||||||||
|
Sudan |
48 |
213.05 (157.09–282.47) |
11 |
47.66 (23.79–85.28) |
59 |
129.36 (98.47–166.86) |
|||||||||
|
Egypt |
15 |
162.87 (91.16–268.62) |
3 |
22.62 (4.67–66.12) |
18 |
80.1 (47.48–126.60) |
|||||||||
|
Philippines |
6 |
11.78 (4.32–25.64) |
10 |
11.77 (5.64–21.64) |
16 |
11.77 (6.73–19.12) |
|||||||||
|
India |
4 |
7.10 (1.94–18.18) |
11 |
9.19 (4.59–16.45) |
15 |
8.52 (4.77–14.06) |
|||||||||
|
Others |
40 |
|
57 |
|
97 |
|
|||||||||
|
|
|||||||||||||||
|
Total overseas born |
151 |
11.52 (9.76–13.51) |
143 |
8.12 (6.84–9.57) |
294 |
9.57 (8.51–10.73) |
|||||||||
|
|
|||||||||||||||
|
* Average over 10-year study period. |
|||||||||||||||
The treatment of nursing home-acquired pneumonia using a medically intensive Hospital in the Home service
Nursing home-acquired pneumonia (NHAP) is a common cause of admissions to hospital of people living in residential aged care facilities (RACFs); most studies have found that it accounts for the majority of non-trauma-related acute hospital admissions in this population.1–9 Dementia, immobility, swallowing problems, diabetes and renal impairment are typical comorbidities;9–11 disease severity on presentation is therefore typically greater than for community-acquired pneumonia, and in-hospital and 30-day mortality rates are high, ranging from 10% to 40%.4,9–15 NHAP requires significant inpatient resources, as the hospital length of stay for these admissions is higher than for community-acquired pneumonia, with published mean stays of between 7.0 and 18.7 days.9–15 Morbidity is also high in this group of patients, who are exposed to increased rates of unintended harm from complications such as falls and pressure wounds.9–17
Nevertheless, most NHAP patients are treated successfully and discharged back to their usual care.7,8,18 Despite growing concerns, most respiratory infections in this group do not involve treatment-resistant organisms.9–15
Some studies suggest that NHAP mortality does not differ between those treated in nursing homes and those transferred to hospital.19,20 However, these studies may not adequately match patients with regard to severity of disease, and it is difficult to ascertain the level of intervention delivered to NHAP patients.21,22 Oral therapy (antibiotics, rehydration) is unlikely to be tolerated by an unwell RACF patient with pneumonia.
Families and patients accept alternatives to hospitalisation that deliver similar care to hospitals.23 Hospital in the Home (HITH) has been established in Victoria for 20 years. This model of care has been shown to be safe and efficacious, including by a randomised study of mild to moderately severe community-acquired pneumonia.24,25 Its use has also been described in the acute management of older patients, and of patients in nursing homes.26–28 However, we could find no published study that specifically examined the safety and outcome of NHAP treated in a medically managed HITH care model. In July 2013, the Royal Melbourne Hospital established a mobile x-ray service that enhanced the capacity of the HITH unit to diagnose and treat patients with suspected significant lower respiratory tract infections in their nursing homes.
The aim of this study was to compare the clinical characteristics, intravenous therapy, treatment safety and outcomes of NHAP treated solely by HITH in the nursing home setting with those of conventional in-hospital treatment.
Methods
The Hospital in the Home intervention model
The Royal Melbourne Hospital HITH service is responsible for all acute medical and pharmaceutical care, and acute nursing, pathology and radiology services for its patients, who remain inpatients of the hospital during their HITH stay. The HITH model provides a 7-day, 24-hour service, can administer oxygen and intravenous antibiotics and fluids, and includes pathology and mobile radiology services. Medical staff visit the patient in their residential home or facility each day, and one or two daily nursing visits are also made.
Study population
This study was a case–control study of patients in high-level RACFs who had been admitted to the Royal Melbourne Hospital for NHAP between 1 July 2013 and 31 January 2014. Patients who had been treated in hospital for more than 48 hours before their admission to the HITH unit were excluded. The study population included patients treated for acute lower respiratory tract infection (pneumonia, aspiration pneumonia, infective exacerbation of chronic obstructive pulmonary disease, infective exacerbation of bronchiectasis), as identified in the database of the HITH unit. The control group included patients with the same diagnoses, but admitted to hospital for conventional care during the study period; they were identified by coding and patient address data recorded by the Health Information Service of the Royal Melbourne Hospital.
Data
Data were obtained from hospital medical records, pathology and radiology systems, and the mobile x-ray service. Patient demographic (age, sex), comorbidity (age-adjusted Charlson comorbidity index), functional status (modified Barthel index) and Mini-Mental State Examination data (if available) were collected. Clinical features were described using the McGeer criteria, and the following laboratory data collected: white blood cell count, haematocrit, C-reactive protein, electrolyte and blood gas levels, and microbiological findings. Pneumonia severity scores (CURB-65, CORB) were calculated from clinical observations and pathology at admission. Information on antibiotic allergies, dehydration, acute confusion, intensive care unit admission, inotropic support, non-invasive ventilation, active resuscitation attempts and advanced care directives was also obtained from records for patients in both groups.
Treatment information gathered from hospital drug charts included the requirement for intravenous fluids or supplemental oxygen, and the duration of intravenous antibiotic therapy. An allergy mismatch was recorded when a patient was prescribed a drug to which an allergy had been noted in their record.
Outcome measures
The primary outcome measure was hospital length of stay. Acute length of stay refers to the stay in the acute ward or HITH; total length of stay refers to the total hospital stay, including acute care and any in-hospital rehabilitation or assessment, such as geriatric evaluation and management.
Secondary outcome measures included 30-day mortality, inpatient mortality, complications, and readmission to hospital within 30 days. These data were obtained from the medical records of the hospital and the RACFs. Complications were classified as treatment-related (antibiotic drug-related adverse events, catheter-related infections) or hospitalisation-related (reported falls, new pressure wounds).
Unplanned interruption refers to a resumed admission to the traditional ward during the HITH admission, where such a return had not been expected or arranged for a procedure or other intervention. This is a clinical quality indicator that is routinely collected by the HITH.
Statistical analyses
This study was designed a priori as an equivalence analysis. It was powered to explore the hypothesis that there was no difference between the length of stay in either direction for HITH and control patients, presuming an equivalence limit of 4.5 days. This limit was selected on the basis of published studies which suggest that the mean length of stay for traditional hospital admissions for NHAP ranges between 7.0 and 18.7 days.10–18 Using the conservative estimate of a standard deviation of 8 days, derived from the same papers and our own pilot data, it was estimated that a minimum sample size of 52 patients per group was required to attain a power of 80% for excluding a difference in mean length of stay of greater than 5 days, based on non-overlap of two-sided confidence intervals.
Categorical variables were summarised as frequencies and percentages, and compared using χ2 or Fisher exact tests as appropriate. Continuous variables were summarised as means and SDs or medians and interquartile ranges (IQRs), and compared using t– or Wilcoxon rank-sum tests as appropriate. Length of stay data were compared using median quantile regression, fully adjusted for baseline differences. Logistic regression was used to study 30-day mortality and readmission by group, adjusted for baseline differences. Appropriate goodness-of-fit tests were conducted for regression models.29 For all analyses, P < 0.05 was defined as statistically significant. All analyses were conducted with Stata 13 (StataCorp).
Ethics approval
Approval for the study was obtained from Royal Melbourne Hospital Ethics Committee (reference 2014050).
Results
Sixty HITH patients and 54 hospital control patients were identified during the study period. The HITH patients were older (P = 0.042), were less likely to have an advanced care plan at the time of treatment (P < 0.001), were more likely to be dehydrated (P = 0.06), and were less likely to have received non-invasive ventilation support (P = 0.01); the group included more men than women (P = 0.026) (Box 1). There were no other significant differences in baseline characteristics between the two groups. Thirty-two patients (53%) were admitted directly into HITH care without a hospital or emergency department stay; 25 (42%) had been referred directly from the emergency department, and three (5%) were referred from the hospital’s rapid medical assessment unit.
Length of stay
There was no difference between the median lengths of stay of HITH and control patients after adjusting for baseline differences (median regression coefficient, −1.00 days; 95% CI, −2.72 to 0.72; P = 0.252).
Management of NHAP
There was no significant difference between the proportions of patients in the two groups for whom blood tests and chest x-rays were undertaken. Microbiological specimens were submitted for a significantly greater proportion of patients in the control group (P < 0.001), but there was no difference in the rate of detection of specific respiratory pathogens. Similar proportions of patients in the two groups were given intravenous fluids and supplemental oxygen. Non-invasive ventilation was more likely to be used in the control group (it was not used for HITH patients). No patient from either group was mechanically ventilated. The duration of intravenous antibiotic treatment before switching to oral antibiotics was significantly longer for HITH patients (P < 0.001) (Box 2).
The pattern of antibiotic prescribing is summarised in Box 3. HITH patients received ceftriaxone and moxifloxacin more often than control patients, who were more likely to receive azithromycin and metronidazole.
The frequency of antibiotic allergy mismatch in the two groups was similar. All except one of the mismatch cases involved mild or non-life-threatening mismatches. There were no reported adverse reactions associated with antibiotic treatment.
Mortality
There were no significant differences in overall mortality at 30 days for the two groups, after adjusting for baseline differences (adjusted odds ratio [aOR] for HITH v control patients, 1.97; 95% CI, 0.67–5.73). Inpatient mortality for HITH patients was lower (aOR, 0.19; 95% CI, 0.05–0.75), but unadjusted postdischarge 30-day mortality was higher than for the control group (OR, 13.25; 95% CI, 1.67–105.75). The number of deaths by 30 days after discharge (12 HITH patients, one control patient), however, limited logistic modelling to unadjusted regression, as indicated by the broad confidence interval. There were no differences between the two groups with regard to complications and 30-day readmission rates after adjusting for baseline differences (aOR, 1.59; 95% CI, 0.30–8.53) (Box 4).
One HITH patient had to return to hospital because the nursing home withdrew their consent for HITH treatment, despite the continuing consent of the family.
Discussion
This study describes the differences between the treatment of NHAP in a traditional hospital setting and when wholly managed in a medically intense HITH setting supported by a mobile x-ray service. There was evidence that the severity of the HITH cases of NHAP was similar to that of cases admitted to hospital with this condition, and our results suggest that the length of stay for these two treatment options may be comparable. It was also found that, after adjustment for baseline differences, there were no significant differences between the 30-day mortality rates and those of readmission to hospital for the two approaches. This suggests that the HITH model is effective and safe for this group of patients. The outcomes we report are consistent with those reported in the international literature.
The median length of stay (reported instead of the mean because of the skewed nature of the data) was shorter than expected in both groups, but the mean total hospital length of stay for the control group was within the range reported for NHAP by other studies.9–15
There are several possible explanations for the finding that in-hospital mortality among HITH patients was lower. First, traditional hospitals may be more forceful in applying rigid limits to the duration of treatment, withdrawing active therapy earlier than in the HITH setting. Prolonging intravenous antibiotic treatment for HITH cases may improve short-term clinical responses. Second, the hospital generally provides palliation services until the death of the patient, while HITH patients were often discharged to their usual care providers after active treatment was stopped and palliation had begun. Finally, the process of transfer to hospital may accelerate clinical deterioration in this frail group.
There are potential challenges to the validity of our findings. Despite the matching of patients and adjustment for baseline differences, this was not a randomised study. We cannot, therefore, exclude the possibility that the two groups differed with regard to other variables, known or unknown, that could account for our findings. Selection of a patient into the HITH group may have been based on the awareness of HITH on the part of the attending doctor or the referring nursing home, the wishes of the patient or their family, or may have been influenced by the availability of a hospital bed at the time of presentation. The reasons for not transferring a patient to HITH in this study could include family reluctance to consider an alternative to traditional hospital care; the unwillingness of the patient’s RACF to accept HITH, or a lack of awareness or will on the part of the treating emergency department doctors of HITH; further unknown or unmeasured markers of disease severity; or the time of presentation. The cluster of RACFs that allowed HITH may have influenced the outcomes (although only one facility refused ongoing HITH, and then only once). These factors may all have influenced the outcomes measured by our study, and caution is required in their interpretation.
HITH offers a partnership in which the RACF provides the sick resident with the usual supportive care, while the HITH provides acute hospital-level intervention. A high level of care was provided: daily medical visits from doctors employed by the hospital; in most cases, twice daily visits by nurses; intravenous fluids and antibiotic treatment, blood tests and x-rays, and 24-hour cover. This study found only one episode where HITH care was terminated by the nursing home before completion, and none in which it was ended by the family. This might suggest that families and the RACFs found the intervention acceptable, but we did not specifically measure satisfaction in this study.
The global impact of NHAP is sobering: there are more than 4 million cases annually, at a median rate of 1.0–3.2 cases per 1000 bed-days and 600 000 emergency admissions.9,11 In 2013, the Australian Institute of Health and Welfare found that permanent RACF patients accounted for 31 760 acute hospital admissions for pneumonia in Australia during the 2008–09 financial year.30 The authors of the report assumed a mean hospital length of stay of 7 days for pneumonia, which amounts to 222 320 hospital bed-days for 2008–09, or 609 fully occupied Australian hospital beds for patients with NHAP. The potential impact of moving some of those patients into HITH care would be substantial.
It is a challenge for hospitals to reconsider the best place to deliver acute care for specific segments of the population. Simply reducing hospital stays for severely unwell patients is not always acceptable. Systems are designed around traditional admission procedures, and alternatives are often not sufficiently prominent or adequately resourced to make a significant impression on the usual processes. However, the proportion of the hospital workload associated with treating RACF patients will only increase. HITH may provide a targeted and effective hospital response that can deliver equivalent quality care without extending the patient’s length of stay. This requires well resourced, intensive, medically based HITH, supported by hospital-level technologies, such as intravenous therapies, expert staff and mobile x-ray facilities, as well as the willingness to meet the challenge of switching care models for the high level of disease severity with which these patients inevitably present.
Box 1 –
Demographic and baseline characteristics of patients in the Hospital in the Home and control groups
|
Hospital in the Home patients |
Control patients |
P |
|||||||||||||
|
|
|||||||||||||||
|
Number of patients |
60 |
54 |
|||||||||||||
|
Median age (IQR), years |
86.5 (83–92) |
84 (77–98) |
0.042 |
||||||||||||
|
Sex, male |
37 (62%) |
22 (41%) |
0.026 |
||||||||||||
|
Antibiotic allergy |
17 (28%) |
17 (31%) |
0.714 |
||||||||||||
|
Median age-adjusted Charlson comorbidity index score (IQR) |
7 (6–8.5) |
7 (5–8) |
0.535 |
||||||||||||
|
Advance care directives |
27 (45%) |
42 (78%) |
< 0.001 |
||||||||||||
|
Diagnosis∗ |
|||||||||||||||
|
Pneumonia/acute lower respiratory tract infection |
50 (83%) |
39 (72%) |
0.152 |
||||||||||||
|
Aspiration pneumonia |
10 (17%) |
15 (28%) |
0.152 |
||||||||||||
|
Chronic obstructive pulmonary disease, infective exacerbation |
7 (12%) |
9 (17%) |
0.443 |
||||||||||||
|
CURB-65 score ≥ 3† |
41 (68%) |
41 (76%) |
0.368 |
||||||||||||
|
CORB score ≥ 2‡ |
36 (60%) |
41 (76%) |
0.070 |
||||||||||||
|
Confusion |
43 (72%) |
39 (72%) |
0.947 |
||||||||||||
|
Dehydration |
40 (67%) |
22 (41%) |
0.006 |
||||||||||||
|
Fulfilled McGeer criteria |
|||||||||||||||
|
Pneumonia |
32 (53%) |
30 (56%) |
0.812 |
||||||||||||
|
Lower respiratory tract infection |
27 (45%) |
22 (41%) |
0.646 |
||||||||||||
|
Pneumonia/lower respiratory tract infection |
59 (98%) |
52 (96%) |
0.498 |
||||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range. * Patients could be assigned to more than one diagnostic category. † Three of: confusion; urea >7 mmol/L; respiration >30/min; blood pressure <90 mmHg; age >65 years. ‡ Two of: confusion (acute); oxygen saturation ≤90%; respiration >30/min; blood pressure <90 mmHg (systolic) or <60 mmHg (diastolic). |
|||||||||||||||
Box 2 –
Investigation and management of patients with nursing home-acquired pneumonia
|
Hospital in the Home patients |
Control patients |
P |
|||||||||||||
|
|
|||||||||||||||
|
Number of patients |
60 |
54 |
|||||||||||||
|
Blood tests |
57 (95%) |
53 (98%) |
0.620 |
||||||||||||
|
Radiological investigations |
|||||||||||||||
|
Chest x-ray |
51 (85%) |
52 (96%) |
0.057 |
||||||||||||
|
Presence of consolidation |
32 (53%) |
31 (57%) |
0.662 |
||||||||||||
|
Microbiological investigations |
|||||||||||||||
|
Microbiological specimen sent |
25 (42%) |
39 (72%) |
0.001 |
||||||||||||
|
Respiratory specimen sent |
9 (15%) |
17 (32%) |
0.036 |
||||||||||||
|
Respiratory pathogens identified |
6 (10%) |
6 (11%) |
0.847 |
||||||||||||
|
Intravenous fluids |
50 (83%) |
40 (74%) |
0.226 |
||||||||||||
|
Oxygen supplementation |
41 (68%) |
44 (82%) |
0.108 |
||||||||||||
|
Non-invasive ventilation support |
0 |
6 (11%) |
0.010 |
||||||||||||
|
Median duration (range) of intravenous antibiotic treatment before switch to oral treatment, days |
4 (0–12) |
2 (0–5) |
< 0.001 |
||||||||||||
|
Allergy mismatch |
4 (7%) |
4 (7%) |
1.000 |
||||||||||||
|
Intensive care unit admission |
0 |
0 |
NA |
||||||||||||
|
Inotropic support |
0 |
0 |
NA |
||||||||||||
|
Resuscitation |
0 |
0 |
NA |
||||||||||||
|
|
|||||||||||||||
|
NA = not applicable. |
|||||||||||||||
Box 3 –
Drug selection for initial treatment of patients with nursing home-acquired pneumonia
|
Drug |
Hospital in the Home patients |
Control patients |
P |
||||||||||||
|
|
|||||||||||||||
|
Ceftriaxone |
54 (58%) |
41 (44%) |
0.044 |
||||||||||||
|
Azithromycin |
14 (15%) |
23 (25%) |
0.028 |
||||||||||||
|
Moxifloxacin |
12 (13%) |
1 (1%) |
0.002 |
||||||||||||
|
Metronidazole |
9 (10%) |
15 (16%) |
0.095 |
||||||||||||
|
Benzyl-penicillin |
1 (1%) |
5 (5%) |
0.100 |
||||||||||||
|
Gentamicin |
1 (1%) |
0 |
1.000 |
||||||||||||
|
Vancomycin |
1 (1%) |
3 (3%) |
0.343 |
||||||||||||
|
Cefazolin |
0 |
1 (1%) |
0.474 |
||||||||||||
|
Clindamycin |
0 |
1 (1%) |
0.474 |
||||||||||||
|
Piperacillin/tazobactam |
0 |
2 (2%) |
0.222 |
||||||||||||
|
Meropenem |
0 |
1 (1%) |
0.474 |
||||||||||||
|
|
|||||||||||||||
|
Total prescriptions |
93 |
92 |
|||||||||||||
Box 4 –
Outcomes for patients with nursing home-acquired pneumonia
|
Hospital in the Home patients |
Control patients |
P |
|||||||||||||
|
|
|||||||||||||||
|
Number of patients |
60 |
54 |
|||||||||||||
|
Median acute care length of stay (range), days |
4 (1–12) |
4 (1–28) |
0.959 |
||||||||||||
|
Total length of stay (range), days |
4 (1–12) |
4 (1–81) |
0.841 |
||||||||||||
|
Mean total length of stay in hospital, days |
5 |
10 |
|||||||||||||
|
Complications |
|||||||||||||||
|
Antibiotic-related |
0 |
2 (3.7%) |
0.222 |
||||||||||||
|
Catheter-related |
0 |
0 |
NA |
||||||||||||
|
Falls |
0 |
2 (3.7%) |
0.222 |
||||||||||||
|
Pressure wounds |
0 |
0 |
NA |
||||||||||||
|
Mortality |
|||||||||||||||
|
Inpatient |
4 (6.7%) |
17 (31.5%) |
0.001 |
||||||||||||
|
30-day |
12 (20%) |
1 (1.9%) |
0.002 |
||||||||||||
|
Overall in 30 days |
16 (26.7%) |
18 (33.3%) |
0.437 |
||||||||||||
|
Readmission within 30 days |
6 (10%) |
3 (5.6%) |
0.496 |
||||||||||||
|
Unplanned hospital admission |
1 (1.7%) |
NA |
NA |
||||||||||||
|
|
|||||||||||||||
|
NA = not applicable. |
|||||||||||||||
[Correspondence] Post-authorisation assessment of orphan drugs
The EU regulation of orphan drugs has promoted the development of new treatments for rare disorders.1 However, the high cost of most orphan drugs threatens the sustainability of public health care. Unfortunately, the effectiveness of treatment is often unclear for part, if not all, of the patient population, especially for patients with very rare diseases, such as inherited metabolic disorders. We believe that the system of post-authorisation assessment for orphan drugs needs to be reformed to address these problems.
[Comment] Alcohol burden in low-income and middle-income countries
Alcohol use contributes to roughly 4% of the global burden of disease.1 Episodic (binge) drinking and high average volume consumed both contribute to this burden in complex ways.1 Episodic drinking increases risks of injury and cardiovascular disease; cancer risk increases with average volume; and low–moderate alcohol use is associated with a reduced risk of death from cardiovascular diseases.2,3 A major limitation of the evidence is that most epidemiological studies of alcohol have been done in high-income and middle-income countries.
[Comment] Treatments for rare diseases: molybdenum cofactor deficiency
In The Lancet, Bernd Schwahn and colleagues1 report on the follow-up of a cohort of infants with molybdenum cofactor deficiency (MoCD), some of whom benefited greatly from a novel therapy. A very rare metabolic disorder is perhaps an unlikely subject for a general medical journal, but new treatments for rare metabolic diseases have problems in common that need much thought.
Legionella pneumonia with severe rhabdomyolysis
A 41-year-old man presented with a 5-day history of fever, non-productive cough and shortness of breath associated with copious watery diarrhoea and excessive fatigue. Apart from heavy smoking and alcohol misuse, there was no significant past medical history including no recent travel.
On examination, we found the patient to be diaphoretic, tachycardic (144 beats/min) and tachypnoeic (42 breaths/min), with a temperature of 39.7°C and blood pressure of 155/105 mmHg. He had an oxygen saturation of 98% after 8 L/min of supplemental oxygen. Auscultation of the chest elicited coarse crepitations over the right lung base and normal heart sounds. He had generalised weakness but no focal neurological deficit.
Laboratory results on admission showed a sodium level of 126 mmol/L (reference interval [RI], 137–145 mmol/L); chloride level, 96 mmol/L (RI, 100–109 mmol/L); potassium level, 3.3 mmol/L (RI, 3.5–4.9 mmol/L); urea level, 10.7 mmol/L (RI, 2.7–8.0 mmol/L), rising to a peak of 46.5 mmol/L; and creatinine concentration 310 μmol/L (RI, 50–120 μmol/L), rising to a peak of 908 μmol/L. Liver function test results were moderately abnormal: aspartate transaminase, 471 U/L (RI, < 45 U/L; peak, 860 U/L); alanine transaminase, 200 U/L (RI, < 55 U/L; peak, 1309 U/L); and lactate dehydrogenase, 4379 U/L (RI, 110–230 U/L). There was leucocytosis, with a white blood cell count of 13.6 × 109/L (RI, 4.0–11.0 × 109/L), neutrophilia (87.1%), mild thrombocytopenia (platelet count, 114 × 109/L (RI, 150–400 × 109/L) and an elevated C-reactive protein level (360 mg/L, RI, < 10 mg/L).
The patient’s serum creatine kinase (CK) level was elevated to 89 860 U/L (RI, < 250 U/L) and his serum myoglobin level was 42 268 μg/L (RI, < 85 μg/L). CK levels peaked at 141 116 U/L on Day 3.
On admission, the patient’s chest x-ray showed right middle lobe consolidation. His renal ultrasound was unremarkable. Subsequently, a sputum culture grew Legionella pneumophila serogroup 1. Urinary antigen test results were also positive for this organism, further confirmed by serology (antibody titre of 1 : 128).
The patient was admitted to the intensive care unit and treatment commenced with ceftriaxone 2 g (stopped after serological confirmation of Legionella) and azithromycin 500 mg daily (continued for 14 days). He was oligoanuric and required haemodiafiltration, which improved urine output along with renal functions. The principal cause for his renal failure was postulated to be severe rhabdomyolysis. Although dehydration related to diarrhoea may have been contributory, it was not deemed to be the main cause of renal failure since the patient was never hypotensive during admission. He was discharged from hospital after 20 days, with normal electrolyte levels and near-normal renal function.
Postinfection rhabdomyolysis is a rare complication associated with Legionella, first described in 1980.1 The mechanism of rhabdomyolysis associated with Legionella infection is unknown. Theories include direct invasion of Legionella into the muscle itself, or release of its endotoxin into the circulation with subsequent muscle injury.2
A literature review identified only 23 cases of rhabdomyolysis associated with Legionella infection. Most cases were from non-English publications, which could not be adequately translated, limiting data extraction. A CK level increase of > 50 000 U/L was identified in only seven of the 23 patients (Box).3–9 A marked predominance of male sex was observed. All patients underwent dialysis except one, who was managed with intravenous mannitol and forced alkaline diuresis,3 but all survived.
In conclusion, while a moderate increase in CK concentration is common with Legionella infection, marked rhabdomyolysis with CK levels > 50 000 U/L leading to acute renal failure is rare. To our knowledge, this is the first case report of L. pneumophila from Australia with this association. Clinicians should be aware of this possible complication and maintain a high index of suspicion, since early recognition and prompt treatment will prove lifesaving.
Box –
Seven out of 23 reported cases had creatine kinase (CK) levels higher than 50 000 U/L3–9
|
Patient no. |
Year, journal |
Patient age in years |
Sex |
Location |
Creatinine at presentation (μmol/L) |
Maximum CK (U/L) |
Dialysis |
Outcome |
|||||||
|
|
|||||||||||||||
|
1 |
1983, Chest |
26 |
Male |
United States |
203 |
165 600 |
Yes |
Survived |
|||||||
|
2 |
1997, Enfermedades Infecciosas y Microbiología Clínica |
61 |
Male |
France |
91 |
202 900 |
No |
Survived |
|||||||
|
3 |
2002, Southern Medical Journal |
56 |
Male |
United States |
247 |
115 880 |
Yes |
Survived |
|||||||
|
4 |
2007, New York Medical Journal |
62 |
Male |
United States |
318 |
176 526 |
Yes |
Survived |
|||||||
|
5 |
2008, Journal of Clinical Pathology |
54 |
Male |
Japan |
450 |
52 000 |
Yes |
Survived |
|||||||
|
6 |
2011, Journal of Cardiology Cases |
58 |
Male |
Japan |
300 |
93 320 |
Yes |
Survived |
|||||||
|
7 |
2012, Chest |
42 |
Male |
United States |
167 |
110 355 |
Yes |
Survived |
|||||||
|
|
|||||||||||||||
News briefs
Sonic “tractor beam” could have medical uses
The tractor beam, a Star Trek staple, could be about to happen, and there could be medical applications, report The Japan Times and The Guardian. Researchers from the University of Bristol in the UK, and Spain’s Public University of Navarre say they have developed a tractor beam that “uses high-amplitude sound waves [at a frequency of 40 kilohertz] to levitate, move and rotate small objects without making contact with them”. The waves took the form of “tweezers to lift an object, a vortex to hold a levitating object in place and a cage to surround an object and hold it in place”. “Sound cannot travel through the void of space, but it can do it through water or human tissue. This potentially enables the manipulation of clots, kidney stones, drug capsules, microsurgical instruments or cells inside our body without any incision,” one of the lead researchers said.
Two-thirds of the world’s under 50s have herpes
The World Health Organization reports that more than 3.7 billion people under the age of 50 – or 67% of the population – are infected with herpes simplex virus type 1 (HSV-1). “Some 140 million people aged 15-49 years are infected with genital HSV-1 infection, primarily in the Americas, Europe and Western Pacific”, WHO says. “Fewer people in high-income countries are becoming infected with HSV-1 as children, likely due to better hygiene and living conditions, and are instead at risk of contracting it genitally through oral sex after they become sexually active.” WHO estimated that 417 million people aged 15-49 years have HSV-2 infection, which causes genital herpes. Taken together, the estimates reveal that over half a billion people between the ages of 15-49 years have genital infection caused by either HSV-1 or HSV-2.
23andMe is back in business
Two years after it was banned from distributing health information to its customers, controversial health and ancestry information provider 23andMe is back in business, reports Gizmodo Australia. In 2013, the US’s Food and Drug Administration stopped the company from providing private customers with health and ancestry information directly from their sequenced DNA, saying it was “concerned about the public health consequences of inaccurate results from the [23andMe] device … the main purpose of compliance with FDA’s regulatory requirements is to ensure that the tests work”. Now the FDA has given 23andMe the green light to resume distributing health information, albeit in a more limited way. “The new reports will provide details about what’s known as ‘carrier status’. The tests will identify genetic mutations in DNA samples that could lead to the passing of one of 36 diseases — including cystic fibrosis, sickle cell anaemia and Tay-Sachs — on to offspring. In each case, the disease would only be passed on if both parents shared the same mutation and the child inherited both mutated genes.” 23andMe has also hiked prices from USD$99 to USD$199.
Can Google Glass help autistic kids?
Wired reports that researchers at Stanford University in the US are working on software for Google’s wearable computer, Glass, which will help autistic children recognise and understand facial expressions and, through them, emotions. Lead researcher Catalin Voss has previously developed a Glass app which recognises emotions, which is now being turned into heads-up technology for cars. The new app is designed like an interactive game. “Children are asked to, say, find someone who is happy”, the researchers said. “When they look at someone who is smiling, the app recognises this and awards points. You can plot, as they wear the glasses, how they’re improving, where they’re improving. You can look at video to understand why.” The app is now being tested in a clinical trial with 100 children.
“Flakka” worse than ice, says toxicologist
A synthetic drug considered fatal has been detected in Australia and has the potential to be worse that ice, the International Business Times reports. “Flakka” is man-made, “has a similarity to cocaine and can be injected, snorted or smoked”. It can lead to a series of extreme symptoms called “excited delirium”, marked by violent behaviour, paranoia and spikes in body temperature. Reports from the United States suggest flakka, also known as “gravel” has caused several deaths there. “Flakka comes in bulk from China and is sold through gas stations, via the internet and other dealers”. Forensic toxicologist Andrew Leibie said that the drug has become so popular with people that “it will be appearing on the streets, it will be appearing in schools, it will be appearing in workplaces.”

 more_vert
more_vert