In addition to morbidity and premature mortality, physical inactivity is responsible for a substantial economic burden. This paper provides further justification to prioritise promotion of regular physical activity worldwide as part of a comprehensive strategy to reduce non-communicable diseases.
Preference: Infectious Diseases and Parasitology
434
The prevalence and clinical associations of HTLV-1 infection in a remote Indigenous community
The known The human T-lymphotropic virus type 1 (HTLV-1) is endemic to central Australia according to hospital and laboratory data for Indigenous adults admitted to Alice Springs Hospital. However, the data may underestimate the overall community prevalence of HTLV-1 infection in remote communities.
The new The prevalence of HTLV-1 infection in a remote Northern Territory community was high: 30 of 74 adults tested were HTLV-1-positive, and nine had clinical syndromes potentially attributable to HTLV-1 infection.
The implications HTLV-1 infection may be more prevalent among Indigenous Australians and be associated with a greater burden of clinical disease than is currently appreciated.
The human T-lymphotropic virus type 1 (HTLV-1) is an oncogenic retrovirus that preferentially infects CD4+ T-cells.1 Worldwide, at least 5–10 million people are infected with HTLV-1, most dwelling in areas of high endemicity in southern Japan, the Caribbean basin, South America or intertropical Africa.2 Transmission typically follows exposure to infected lymphocytes in blood, or through breastfeeding or sexual intercourse. A minority of people infected with HTLV-1 experience a rapidly progressive haematological malignancy (adult T-cell leukaemia/lymphoma [ATLL])3 or inflammatory disorders4 such as HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP)5 and HTLV-1-associated pulmonary disease.4,6 Although the lifetime disease-specific risks for HAM/TSP and ATLL in Japan and the Caribbean are low (0.3–1.9%7,8 and 1–5%1 respectively), the true burden of HTLV-1-associated diseases has not been determined in a community setting.
HTLV-1 was first identified in central Australia in 19889 and each of the major recognised complications of HTLV-1 have since been described in Indigenous residents of this region.3,5,10,11 HTLV-1-associated pulmonary disease is particularly common6,10,12 and contributes to the highest reported adult prevalence of bronchiectasis worldwide.6 An impaired immune response also contributes to HTLV-1-associated morbidity by increasing the larval burden of Strongyloides stercoralis in people infected with HTLV-1 living in resource-poor areas.1,10 Notwithstanding seropositivity rates that exceed 30% for Indigenous adults admitted to Alice Springs Hospital,10 there has been no coordinated program to reduce viral transmission among Indigenous Australians.
The development of strategies for controlling HTLV-1 transmission in Australia is hampered by limitations of the epidemiological data. Indeed, although the HTLV-1c subtype is thought to be endemic to central Australia, this conclusion is based on laboratory data for Indigenous adults admitted to Alice Springs Hospital,2 and these data may substantially underestimate the prevalence of HTLV-1 infections in some Indigenous communities. Conversely, only two studies have reported community-based seropositivity rates, for 3613 and 1319 Indigenous people from undefined populations. In endemic areas, such as southwestern Japan, mother-to-child transmission is thought to be the primary mode of transmission,14 and this is also assumed to be the case in central Australia.15 Nevertheless, HTLV-1 testing is not currently included in routine antenatal screening.16 Modes of transmission other than breastfeeding may be important for Indigenous Australians, among whom infection rates are highest for hospitalised men.10
Community-based studies are essential for defining the epidemiological associations of HTLV-1 infection in Australia and for accurately determining its prevalence. Such work is complicated by the historical burden of mistrust associated with research in Indigenous communities.17 Here we report the results of a community-based survey of HTLV-1 infection using a culturally safe model that integrated clinical research with a health literacy program; it is the first such community-based survey to be reported. In addition to determining HTLV-1 seroprevalence and its clinical associations, we quantified the number of infected peripheral blood cells with a recently developed HTLV-1c proviral load (PVL) test. A higher HTLV-1 PVL is associated with ATLL and HAM/TSP,1 but data on HTLV-1c PVL are limited, and have not previously been reported for a community-based survey.
Methods
A single remote Northern Territory Indigenous community (estimated resident population [2015], 138) was selected for our pilot study, conducted during two visits, 25–29 August 2014 and 15–19 June 2015.
Phase 1: community engagement
A knowledge translation process and health literacy resources were developed during visits to the community by an Aboriginal research officer (CP) and a non-Indigenous academic (KT). These resources were used by a clinician (LE) during two subsequent visits for discussing (through an interpreter, CP) the major disease associations and the risk of HTLV-1 transmission by sexual contact or exposure to infected blood. Given current uncertainties about the predominant mode of transmission and the clear benefit of breastfeeding in remote Indigenous communities, mother-to-child transmission was not discussed. Health messages were provided separately to men and women.
Phase 2: clinical survey
Male and female Aboriginal team members again provided health literacy information. Individuals were then invited to participate in the study, and consent was obtained in their primary language. The clinical survey incorporated a health questionnaire and a limited physical examination. Data collected included self-reported comorbid conditions and respiratory, gastrointestinal, dermatological and neurological symptoms. All surveys were performed while blinded to the HTLV-1 serostatus of the participants. Clinical records were subsequently reviewed to confirm comorbidity data. Physical examination was restricted to a respiratory examination and limited neurological (gait and lower limb tone, power, reflexes and sensation) and dermatological examinations (generally restricted to the head, back, limbs and abdomen).
Whole blood samples were collected into EDTA-coated tubes. Samples were processed at Alice Springs Hospital and forwarded for Strongyloides serological testing (Western Diagnostic Pathology, Perth). Peripheral blood buffy coats were recovered and stored at −70°C for HTLV-1 studies. Blood was not collected from children under 2 years of age.
Community residence was determined from the community health clinic registry. Chronic lung disease was defined as a daily productive cough (lasting at least one month) with inspiratory crackles on auscultation of the chest. Diarrhoea was defined as the passing of several loose stools each day for more than 2 days.
HTLV-1 serologic and molecular studies
Analyses were performed at the National Serology Reference Laboratory, Melbourne, blinded to the clinical state of participants. Plasma HTLV-1 antibodies were detected by enzyme immunoassay (Murex HTLV I + II, DiaSorin) and titres determined with a twofold endpoint dilution method by particle agglutination (Serodia-HTLV-1, Fujirebio). All samples reactive on either screening assay were confirmed by western blot (WB) (HTLV-I/II Blot2.4, MP Diagnostics). HTLV-1 infection was defined by a positive WB test result.
HTLV-1 PVL was determined by polymerase chain reaction (PCR) using primers and probes that targeted a highly conserved region at the 5′ end of the gag gene in the p19 coding region of HTLV-1c (Mel5; accession number, L02534). SP cells18 containing a single integrated, full-length copy of HTLV-1 and one copy of the albumin gene were used to generate a standard curve for determining HTLV-1 copy numbers and cell numbers. The number of HTLV-1 copies per peripheral blood leucocyte (PBL) was then calculated. PVL was expressed as HTLV-1 copies/105 PBL. The lower limit of detection was 6.5 copies for HTLV-1 (95% confidence interval [CI], 5.4–8.4) and 15.6 for albumin (95% CI, 12.9–20.0).
Statistical analysis
Categorical variables were compared in χ2 or Fisher exact tests as appropriate. Continuous variables were assessed for significant departures from normality. Normally distributed variables were summarised as means and standard deviations, and compared in t tests. Variables with skewed distributions were summarised as medians and interquartile ranges (IQRs), and compared in Wilcoxon rank-sum tests. All analyses were performed in Stata 14.0 (StataCorp).
Ethics approval
The study was developed in collaboration with Primary Health Care Remote, Central Australia Health Service, Alice Springs. All participants with a clinical condition were referred for appropriate follow-up and treatment. The study was approved by the Central Australian Human Research Ethics Committee (reference, HREC-14-242).
Results
One hundred and four people (75% of the estimated resident population) consented to participate in the study. Blood was not collected from four children, one sample was lost in transit, and a 68-year-old man with chronic lung disease and ataxia declined to give blood. HTLV-1 test results were discordant (PCR-positive, WB-negative) for an 11-year-old boy with crusted scabies whose mother and maternal grandmother were infected with HTLV-1, and he was excluded from the study. The final analysis therefore included 97 participants (70% of the resident population): 23 children (13 boys, 10 girls) and 74 adults (39 men, 35 women).
HTLV-1 seropositivity
HTLV-1 seropositivity rates were significantly higher among adults than among children (30 of 74 v 1 of 23; P = 0.001) (Box 1). Rates were highest among adults aged 35 years or more; ten of the 15 men in this age group and seven of the 16 women were infected with HTLV-1 (Box 1).
Clinical associations
Four adult participants did not wait for clinical review; data are therefore presented for 27 adults infected with HTLV-1 and 43 who were not (Box 2). Although half of all adults reported respiratory symptoms, with eight exceptions these were all acute conditions, without clinical evidence of chronic lung disease. A chronic productive cough with physical signs was found in seven adults (four men, three women), each of whom was HTLV-1-positive (Box 2). Four had never smoked, two were current smokers, and one had a past history of smoking. Bronchiectasis was radiologically confirmed in one 52-year-old man and in two women aged 36 and 54 years by chest high resolution computed tomography (cHRCT); the other four HTLV-1-positive adults with chronic lung disease were not examined with cHRCT. A chronic productive cough with physical signs was found in only one (HTLV-1-seronegative) child; bronchiectasis was not apparent on cHRCT, and a diagnosis was made of chronic suppurative lung disease with reactive airway of uncertain aetiology.
The gait of three adults (two women aged 54 and 71 years and a 48-year-old man) was ataxic. All were HTLV-1-positive and had chronic lung disease. The two women were unable to walk without walking aids. Lower limb power and sensation were normal, and there was no increased muscular tone or hyperreflexia.
All five participants who reported diarrhoea were HTLV-1-positive (Box 2); serological results for Strongyloides were positive in two instances. Rates of Strongyloides seropositivity were not significantly different for HTLV-1-positive and -negative adults (HTLV-1-positive, 6 of 28; HTLV-1-negative, 4 of 44; P = 0.172). Three of 15 children tested, including the child with chronic lung disease, were seropositive for Strongyloides and HTLV-1-negative. No participant presented with infective dermatitis, and the only one with crusted scabies was the 11-year-old boy described above.
HTLV-1 proviral load
The HTLV-1 PVL for one WB-positive participant could not be determined for technical reasons. The median HTLV-1 PVL for the remaining 30 HTLV-1-positive participants was 145 (IQR, 16.3–558) copies/105 PBL. The results for three HTLV-1-positive participants who did not wait for clinical examination and for two with symptomatic strongyloidiasis were excluded from further analysis. There was no sex difference in the median HTLV-1 PVL for nine men and eight women who did not present with chronic lung disease, ataxia or strongyloidiasis (men, 51 [IQR, 39–271]) copies/105 PBL; women, 8.6 [IQR, 0.27–179]) copies/105 PBL; P = 0.180). The median HTLV-1 PVL was significantly higher for the seven adults with chronic lung disease than for 17 asymptomatic participants (chronic lung disease, 649 copies/105 PBL [IQR, 162–2220]; asymptomatic adults, 40 copies/105 PBL [IQR, 0.9–229]; P = 0.017) (Box 3). HTLV-1 PVL for each of the three participants with both chronic lung disease and gait ataxia exceeded 1000 copies/105 PBL (1365, 2214 and 3399 copies/105 PBL).
Discussion
We found high rates of HTLV-1 infection among Indigenous adults in a remote Indigenous Australian community. The adult seroprevalence rate in this community (30 of 74 people) is comparable with that of some villages in southwestern Japan prior to public health interventions in that country.19 In contrast to the Japanese results for children, only a single Indigenous child was definitely infected with HTLV-1, and we were unable to test his mother or siblings, as they were absent from the community during the survey. Horizontal transmission may therefore be particularly important in central Australia, as in Jamaica20 and some African countries21 where sexual transmission is the predominant mode. Although our data were derived from a single, small community, HTLV-1 serostatus was determined for 70% of its residents, and the adult seroprevalence rate was very close to that for hospitalised adults from this region.10 Published community-based epidemiological data for HTLV-1 infection in Australia are otherwise limited. HTLV-1 seropositivity rates of 14–15% have been reported for samples of 1319 and 3613 Indigenous residents of central Australia, but the manner in which the participants were selected and the size of the populations from which they were drawn is unclear.
The high rate of HTLV-1 infection in our study community was associated with possibly HTLV-1-linked conditions in nine of 30 HTLV-1-positive adults (chronic lung disease, seven participants; symptomatic strongyloidiasis, two). Seven HTLV-1-positive adults satisfied the case definition for chronic lung disease, and in three instances bronchiectasis was radiologically confirmed. In central Australia, HTLV-1 infection increases the rates of hospitalisation for bronchiectasis, lower respiratory tract infections and asthma;10 in a case–control study, the risk of bronchiectasis was almost twice as high for HTLV-1-infected Indigenous Australians as for uninfected residents.12
Bronchiectasis caused by HTLV-1 is associated with a higher HTLV-1c PVL, and in our study this was also true for participants with chronic lung disease. We have previously reported that HTLV-1 PVL is correlated with the extent of radiologically defined pulmonary injury.10 These observations are consistent with the proposed immunopathology of HTLV-1-linked inflammatory diseases, which are thought to result from an immune response to a high HTLV-1 antigen load.22 In a Japanese cohort, an HTLV-1 PVL of greater than 1000 copies/105 peripheral blood mononuclear cells was predictive of HAM/TSP, and half of all those with this condition had abnormal chest x-rays.23 Gait disturbance is a common manifestation of HTLV-1-associated neurological disease.4 In our study, concurrent gait ataxia and chronic lung disease affected three participants whose HTLV-1 PVL exceeded 1000 copies/105 PBL, and this may reflect the systemic nature of the HTLV-1-mediated inflammatory process.4
The burden of HTLV-1-associated inflammatory diseases has not previously been studied in a community setting. The reported lifetime risk of strictly defined HAM/TSP in people who are HTLV-1-positive ranges between 0.25% for cases solicited from Japanese medical institutions7 to 1.9% based on data provided voluntarily by medical staff to registries in the Caribbean.8 In contrast, neurological and dermatological abnormalities respectively affect more than 30%24 and 70%25 of infected persons followed up in Brazilian outpatient clinics. The true burden of HTLV-1-associated inflammatory disease in a community setting may therefore be far higher than suggested by a strict application of the HAM/TSP case definition to hospitalised patients. Skin diseases were less common among HTLV-1-infected participants in our study than in Brazil.25 This may reflect selection bias in the hospital-based Brazilian cohort, but genetic factors may also contribute to the risk of HTLV-1-induced inflammation in particular organ systems.4
Notwithstanding the success with which we were able to engage with residents of the remote Indigenous community, the study design had a number of limitations. For example, the small sample size precluded a detailed epidemiological analysis that could identify the major modes of HTLV-1 transmission. We were also unable to develop a multivariable model that included other factors that contribute to chronic lung disease, such as smoking and age. A further limitation was our inability to determine the pathological basis for most cases of chronic lung disease and gait ataxia; this would require investigations that are unavailable in a remote community. Similarly, strongyloidiasis was diagnosed serologically because we were unable to collect stool samples prior to treatment. Nevertheless, even if all 18 adult residents not recruited to our study were not infected with HTLV-1, the adult HTLV-1 seroprevalence rate in this community would exceed 30%. Moreover, clinical examinations and HTLV-1 studies were performed without knowledge of HTLV-1 serostatus, and our findings are consistent with studies that have found a strong association between HTLV-1 infection and chronic lung disease in Indigenous Australians.6,10,12
In summary, we found very high HTLV-1 seropositivity rates among adult residents of a remote Indigenous community, and evidence for disease potentially attributable to HTLV-1 in nearly one-third of HTLV-1-positive participants. The project integrated clinical research with a health literacy program, using both Indigenous and non-Indigenous expertise to decolonise research practice.26 Further investigations will apply the lessons learned from this pilot project to other communities, in order to identify the major modes of HTLV-1 transmission and the associated disease burden in remote Australia.
Box 1 –
Prevalence of HTLV-1 infection among 97 Indigenous Australian residents of a remote Northern Territory community, according to age group
|
Age category |
Male |
Female |
|||||||||||||
|
|
|||||||||||||||
|
Children (1–14 years) |
1 of 13 (7%) |
0 of 10 |
|||||||||||||
|
Adults (15–34 years) |
7 of 24 (29%) |
6 of 19 (32%) |
|||||||||||||
|
Adults (≥ 35 years) |
10 of 15 (67%) |
7 of 16 (44%) |
|||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 2 –
Demographic data for 97 Indigenous Australian residents of a remote Northern Territory community, according to HTLV-1 serostatus
|
|
HTLV-1-positive |
HTLV-1-negative |
P |
||||||||||||
|
|
|||||||||||||||
|
All participants |
|
|
|
||||||||||||
|
Number |
31 |
66 |
|
||||||||||||
|
Age |
|
|
0.001 |
||||||||||||
|
Children |
1 |
22 |
|
||||||||||||
|
Adults |
30 |
44 |
|
||||||||||||
|
Sex |
|
|
|
||||||||||||
|
Children |
1 boy |
12 boys, 10 girls |
0.37 |
||||||||||||
|
Adults |
17 men, 13 women |
22 men, 22 women |
0.57 |
||||||||||||
|
Clinical survey findings (adults only) |
|
|
|
||||||||||||
|
Any history of smoking |
13/30 |
10/44 |
0.06 |
||||||||||||
|
Comorbidities |
|
|
|
||||||||||||
|
Diabetes |
12/30 |
8/44 |
0.038 |
||||||||||||
|
Asthma |
1/30 |
1/44 |
0.78 |
||||||||||||
|
Heart disease |
2/30 |
0/44 |
0.083 |
||||||||||||
|
Chronic liver disease |
0/30 |
1/44 |
0.41 |
||||||||||||
|
Chronic kidney disease |
6/30 |
4/44 |
0.18 |
||||||||||||
|
Symptoms* |
|
|
|
||||||||||||
|
Respiratory† |
21/27 |
25/43 |
0.090 |
||||||||||||
|
Diarrhoea‡ |
5/27 |
0/43 |
0.007 |
||||||||||||
|
Dermatological§ |
5/27 |
3/43 |
0.25 |
||||||||||||
|
Possibly HTLV-1-associated conditions* |
|||||||||||||||
|
Chronic lung disease¶ |
7/27 |
0/43 |
0.001 |
||||||||||||
|
Ataxic gait |
3/27 |
0/43 |
0.032 |
||||||||||||
|
Strongyloides seropositivity** |
6/28 |
4/44 |
0.17 |
||||||||||||
|
Symptomatic strongyloidiasis†† |
2/27 |
0/43 |
0.082 |
||||||||||||
|
|
|||||||||||||||
|
* Three HTLV-1-positive participants and one HTLV-1-negative participant provided blood but did not wait for medical review. † Wheeze, dry cough or dyspnoea. ‡ Loose bowel actions several times per day for longer than 2 days. § Pruritus or rash attributed to scabies (three participants), impetigo (one), tinea corporis (one) or pityriasis versicolor (one). ¶ Daily productive cough with inspiratory crackles audible on auscultation of the chest. ** Strongyloides serological results were not available for all participants. †† Loose bowel actions several times per day for more than 2 days, together with positive Strongyloides serological result. |
|||||||||||||||
Box 3 –
HTLV-1 proviral load (PVL) in 24 HTLV-1-positive adults*
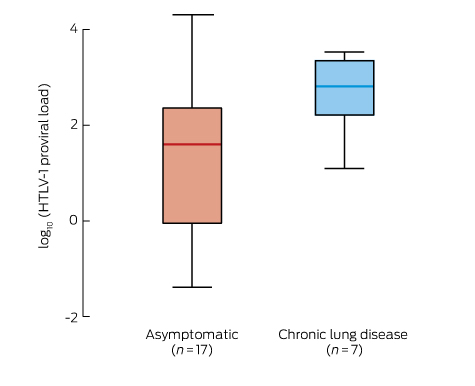
* Expressed as log10(HTLV-1 copies/105 peripheral blood leucocytes). Asymptomatic v chronic lung disease: P = 0.17 (Wilcoxon rank-sum test). Excluded from the analysis were three HTLV-1-positive participants who did not wait for clinical examination, two with symptomatic strongyloidiasis, and one for whom HTLV-1 PVL could not be determined for technical reasons.
No Jab, No Pay — no planning for migrant children
Migration should be considered by immunisation policy
The Social Services Legislation Amendment (No Jab, No Pay) Act 2015 (Cwlth) was passed in November 2015, closing the conscientious objection exemption to immunisation requirements for family assistance payments. The intention was to reinforce the importance of immunisation and protect public health, especially for children.1,2 While these aims are sound, there are far-reaching, presumably unintended, consequences for migrant and refugee children.
The legislative changes (which took effect in January 2016) require children and young people under 20 years of age to be up to date for their early childhood immunisations in order to qualify for the Child Care Benefit, Child Care Rebate and Family Tax Benefit Part A supplement (Box).3 These Centrelink payments are available for Australian citizens and people holding a permanent visa (including offshore humanitarian entrants), special category visa or certain temporary visas (including temporary protection visas). Immunisation status is assessed through the Australian Childhood Immunisation Register (ACIR), which is linked to Medicare.
Medical contraindications (including immunosuppression and anaphylaxis) and natural immunity are still grounds for vaccination exemption. However, the legislation now specifies that only general practitioners can certify exemptions, with the expectation that specialists will refer back to GPs.2 The legislation is paired with a number of supporting measures, including funded catch-up immunisations (time-limited for people aged 10–19 years), expansion of the ACIR to include all people under 20 years of age,4 and provider incentive payments for catch-up vaccination in children aged less than 7 years.5
There are multiple issues arising for refugee and migrant children. First, any child arriving and receiving catch-up vaccination in Australia after the age of 7 years who is eligible for these Centrelink payments will lose them until their ACIR record is updated, even if he or she is fully immunised. Before 1 January 2016, the upper age limit for data entry into the ACIR was 7 years — overseas and catch-up vaccinations could not be recorded on the register for older children. Australia’s Humanitarian Programme intake has been 13 750 people annually, with around 50% aged less than 18 years on arrival.6 Therefore, up to 35 000 refugee children and young people (those who have arrived at the age of 7 years or older and are currently under 20 years of age) will need their vaccination status assessed and ACIR records entered. This number will increase when other migrant children meeting the residency requirements for Centrelink payments are included.
The workforce challenges regarding the No Jab, No Pay measures are substantial. Immunisation providers across Victoria report that refugee families have received (multiple) letters from Centrelink. This has resulted in large numbers of people presenting to services, and an increased demand for providers to clarify previous vaccination history, notify the ACIR of these details, and provide catch-up vaccines where needed. Providers report being inundated, under-prepared and inadequately resourced to meet demand.
Establishing prior vaccination is difficult, time consuming, and may not be possible. Refugee-background families tend to be mobile in the early years of settlement, and often see multiple providers for health care, which may (or may not) include immunisation. Children may receive vaccinations in different parts of the health system — from GPs, from specialists, at school, and, particularly in Victoria, through local government areas (LGAs).7 However, comprehensive records are rare, and information about past vaccinations is often unavailable.
Reporting to the ACIR is time consuming, and there is variation in how information is handled. Providers estimate it takes 20 minutes to enter a full vaccine history into the ACIR online, and longer if overseas vaccinations are recorded. They report delays between submission and registration of data on the ACIR. While on-site vaccines are usually registered within 24 hours, prior vaccines (administered in Australia or overseas) take 1–3 weeks, and individual errors can result in batches of ACIR entries being rejected, affecting ACIR registration for multiple individuals. Many services are now faxing records to the ACIR due to inadequate capacity to enter information directly; these are taking up to 8 weeks to register and delays appear to be increasing. Providers report discrepancies between Centrelink and the ACIR, and cases where families have been sent Centrelink letters, despite children being registered as fully immunised on the ACIR. Paediatricians are the key workforce in childhood immunisation; however, unlike, GPs, they are not automatically registered with the ACIR and the process to obtain or activate specialist ACIR registration is complicated. While specialists may have prescribed catch-up vaccines, they are usually not able to enter this information onto the ACIR, which reduces the opportunity to disseminate the workload and enhance ACIR recording.
Catch-up immunisation generally requires three visits over at least 4 months (four visits over 10 months for children aged 4–9 years), with several vaccines on each visit. Calculating catch-up schedules for migrant children is complex and far more difficult than providing a missed schedule point for an Australian-born child. Primary Health Networks and LGAs report that many GPs feel poorly equipped to deal with this complexity and the time requirements and, in Victoria, are deferring this work to LGAs.
The increase in workload is not reflected in funding arrangements, and the new provider catch-up incentive payments are not structured to support best practice immunisation. Catch-up incentive payments ($6 additional to ACIR notification payments) are only available for children aged less than 7 years, and for vaccines given after 1 January 2016 that are more than 2 months overdue. Thus, if an immunisation provider gives the first doses of a catch-up schedule and recalls the child 1 month later (the minimum interval and best practice), the second vaccinations will not trigger a catch-up incentive payment, as they are not considered to be overdue in relation to the first. Further, the national due and overdue rules in relation to hepatitis B8 are not consistent with the minimum catch-up dosing intervals recommended by the Australian immunisation handbook.9 Hepatitis B vaccination at 0, 1 and 4 months (minimum intervals) will register the child as overdue at the time of the final dose (3 months from previous dose), risking loss of Centrelink payments.
Finally, there is complexity concerning medical contraindications and natural immunity, in that the new legislation specifies that only GPs can provide this information. Many refugee children do not require hepatitis B (or other) vaccines, on the basis of natural immunity from infection or immunity from (undocumented) overseas vaccination. Hepatitis B serology is part of the routine post-arrival refugee health assessment, detecting both infection and immunity. Available Australian data suggest that around 30% of East African and 50% of Karen refugee children have immunity to hepatitis B,10,11 and 2–5% of African children are infected with hepatitis B.12 Many children have thus completed catch-up vaccination without needing hepatitis B (or other) vaccines, but will not be regarded as up to date on the ACIR. They will need a medical exemption form completed by a GP; however, many families have changed GPs in the years after settlement and/or were initially managed and immunised at specialist or nurse-led clinics. GPs will likely be asked to enter historical information on behalf of other providers (which will be almost impossible to verify) and there may be considerable reluctance to do so.
These issues are likely to create duplications within the health system in:
-
appointments — where children had specialist refugee health screening, it is feasible that an LGA may refer children to GPs who may refer them to specialists to clarify immunisation history and serology, who will then refer children back to the GPs for the medical exemption form, who in turn refer them back to the LGA for vaccine delivery;
-
serology — where there is no documentation, GPs and specialists (and families) may choose repeat hepatitis B serology instead of undertaking three immunisation visits; or
-
vaccines — where vaccination history or natural immunity cannot be established.
All these options incur additional costs and represent inefficiencies in the health system.
While the No Jab, No Pay policy offers an opportunity to improve immunisation coverage rates, the legislation will exclude thousands of Australia’s most disadvantaged families from Centrelink payments as a result of system issues rather than any form of conscientious objection. Clinical experience suggests that refugee background families are extremely pro-immunisation, which is consistent with the large numbers presenting to clarify their children’s immunisation status and access catch-up vaccinations. Unfortunately, the legislative and policy changes presume continuity of care, administration of early childhood vaccines during early childhood, prior use of the ACIR, and centralised immunisation delivery, which is not the reality for migrant families.
There are several strategies that could reduce the impact of the No Jab, No Pay measures on migrant children. There is a strong argument to apply the legislation prospectively (to children born 2009 onwards) or to extend the period before Centrelink payments are affected, allowing adequate lead time for entering data into the ACIR and obtain catch-up vaccination if needed. Due and overdue rules and catch-up incentive payments should be structured to support best practice, including removal of the payment age limit. Funding for catch-up vaccinations in those aged 10 years and older should be ongoing, and better resources to support providers, including a whole-of-life calculator and information on refugee immunisation, would increase efficiency and remove barriers to service delivery. Extending the ACIR across the lifespan offers an opportunity to address usability issues and capture relevant demography to monitor immunisation in this group. Finally, authority to document medical exemptions, specialist ACIR registration and workforce pressures require urgent attention. Fundamentally, good policy development should recognise that migration is part of the fabric of Australia, and it is not clear this has been adequately considered in the implementation of No Jab, No Pay.
Box –
Family assistance payments affected by the No Jab, No Pay measures
- Family Tax Benefit Part A (FTB-A) is a two-part payment supporting disadvantaged families with dependent children or secondary students younger than 20 years of age, consisting of an adjusted base rate and a supplement of up to $726.35 per child at the end of the financial year. The maximum adjusted taxable income limits for FTB-A are over $100 000, and it is likely most refugee background families will be eligible for this payment.
- The Child Care Benefit supports costs of registered/approved childcare and outside-school-hour care, with current rates of $4.17 per hour or $208.50 per week (85% for school-aged children), which is income-tested and adjusted for family size, service type and hours attended.
- The Child Care Rebate (non-income-tested) covers 50% of out-of-pocket expenses for childcare to an annual limit for each child, in addition to other childcare assistance.
- Together, these benefits are a substantial support for families with children. For further information, go to https://www.humanservices.gov.au/customer/subjects/payments-families.
[Series] HIV and tuberculosis in prisons in sub-Saharan Africa
Given the dual epidemics of HIV and tuberculosis in sub-Saharan Africa and evidence suggesting a disproportionate burden of these diseases among detainees in the region, we aimed to investigate the epidemiology of HIV and tuberculosis in prison populations, describe services available and challenges to service delivery, and identify priority areas for programmatically relevant research in sub-Saharan African prisons. To this end, we reviewed literature on HIV and tuberculosis in sub-Saharan African prisons published between 2011 and 2015, and identified data from only 24 of the 49 countries in the region.
[Comment] Renewing commitments to physical activity targets in Thailand
The Lancet 2016 Series on physical activity provides global evidence on how physical activity contributes to healthy nations through primary prevention of non-communicable diseases (NCDs), a growing epidemic.1–4 The Series presents compelling evidence on the benefits of physical activity not only for health, but also for social, environmental, and economic outcomes.2,5
[Articles] Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial
Adalimumab significantly lowered the risk of uveitic flare or loss of visual acuity upon corticosteroid withdrawal in patients with inactive, non-infectious intermediate, posterior, or panuveitic uveitis controlled by systemic corticosteroids. No new safety signals were observed and the rate of adverse events was similar between groups. These findings suggest that adalimumab is well tolerated and could be an effective treatment option in this patient population. An open-label extension study (NCT01148225) is ongoing to provide long-term safety data for adalimumab in patients with non-infectious uveitis.
[Comment] GEMS extend understanding of childhood diarrhoea
There is no doubt that the Global Enteric Multicenter Study (GEMS) has not only revolutionised understanding of diarrhoeal diseases in the young and vulnerable in some of the most disadvantaged areas of the world, but it has also altered the standard by which such studies are to be done going forward. Undoubtedly, for data such as these to be meaningful, controls and cases must be included.1,2 GEMS has also elucidated that the poorest of the poor might succumb to different pathogens at different rates in different parts of the world, which potentially affects prioritisation of interventions.
[Comment] Physical activity—time to take it seriously and regularly
In 1994, the epidemiologist Jerry Morris described physical activity as the “best buy” in public health.1 In 2012, The Lancet published its first Series on physical activity, which showed an estimated 5·3 million deaths per year are due to inactivity; the Series concluded that physical inactivity is as important a modifiable risk factor for chronic diseases as obesity and tobacco.2 Today, we publish our second Series, which provides an update of the field since 2012 and an analysis of the latest science of physical activity and health, with a strong focus on low-income and middle-income countries (LMICs).
[Comment] Update on the global pandemic of physical inactivity
Non-communicable diseases (NCDs) are a major burden worldwide. Health behaviours such as tobacco cessation, healthy dietary choices, and low alcohol consumption have all proven effective in the prevention and treatment of NCDs; however, less global attention has been given to the importance of an active lifestyle for disease prevention. In 2012, The Lancet published its first Series on physical activity, which increased awareness of the importance of physical activity in the prevention of NCDs, with a special emphasis on low-income and middle-income countries.
[Department of Error] Department of Error
Nguyen QD, Merrill PT, Jaffe GJ. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet 2016; published online Aug 16. http://dx.doi.org/10.1016/S0140-6736(16)31339-3. In the Methods section of the Summary of this Article, “(aged ≤18 years)” should have been “(aged ≥18 years)”. This correction has been made to the online version as of August 24, 2016.

 more_vert
more_vert