In Hélène Delisle and Malek Batal’s correspondence (June 18, p 2504),1 the authors eloquently noted the dual burdens of undernutrition and overnutrition among socioeconomically disadvantaged populations. However, one important and rapidly increasing group merits further comment. More than 2·5 million Americans abuse heroin or prescription opioids,2 leaving the USA in the midst of an opioid abuse epidemic. The adverse consequences of illicit opioid use (eg, overdose, infectious disease, premature death) are widely discussed.
Preference: Infectious Diseases and Parasitology
434
Association between resting heart rate and coronary artery disease, stroke, sudden death and noncardiovascular diseases: a meta-analysis [Research]
Background:
Resting heart rate is linked to risk of coronary artery disease, stroke, sudden death and noncardiovascular diseases. We conducted a meta-analysis to assess these associations in general populations and in populations of patients with hypertension or diabetes mellitus.
Methods:
We searched PubMed, Embase and MEDLINE from inception to Mar. 5, 2016. We used a random-effects model to combine study-specific relative risks (RRs). We used restricted cubic splines to assess the dose–response relation.
Results:
We included 45 nonrandomized prospective cohort studies in the meta-analysis. The multivariable adjusted RR with an increment of 10 beats/min in resting heart rate was 1.12 (95% confidence interval [CI] 1.09–1.14) for coronary artery disease, 1.05 (95% CI 1.01–1.08) for stroke, 1.12 (95% CI 1.02–1.24) for sudden death, 1.16 (95% CI 1.12–1.21) for noncardiovascular diseases, 1.09 (95% CI 1.06–1.12) for all types of cancer and 1.25 (95% CI 1.17–1.34) for noncardiovascular diseases excluding cancer. All of these relations were linear. In an analysis by category of resting heart rate (< 60 [reference], 60–70, 70–80 and > 80 beats/min), the RRs were 0.99 (95% CI 0.93–1.04), 1.08 (95% CI 1.01–1.16) and 1.30 (95% CI 1.19–1.43), respectively, for coronary artery disease; 1.08 (95% CI 0.98–1.19), 1.11 (95% CI 0.98–1.25) and 1.08 (95% CI 0.93–1.25), respectively, for stroke; and 1.17 (95% CI 0.94–1.46), 1.31 (95% CI 1.12–1.54) and 1.57 (95% CI 1.39–1.77), respectively, for noncardiovascular diseases. After excluding studies involving patients with hypertension or diabetes, we obtained similar results for coronary artery disease, stroke and noncardiovascular diseases, but found no association with sudden death.
Interpretation:
Resting heart rate was an independent predictor of coronary artery disease, stroke, sudden death and noncardiovascular diseases over all of the studies combined. When the analysis included only studies concerning general populations, resting heart rate was not associated with sudden death.
[Perspectives] A laboratory in your pocket
Infectious diseases are a leading cause of death, especially among children in low-income and middle-income countries. Rapid, point-of-care (POC) identification of pathogens could help efforts to control and manage infectious diseases. For most infections, specimen collection and testing is limited to formal medical and laboratory settings. Although rapid diagnostic tests exist for some pathogens, such as influenza, group A streptococcus, and HIV, many still lack specificity and sensitivity; thus, specimens must be sent to a trained and equipped laboratory for gold standard testing.
[World Report] NIH project focuses on integration of HIV and NCD care
The US National Institutes of Health and partner agencies are exploring ways to combine health services for HIV and non-communicable diseases in resource-poor settings. Andrew Green reports.
[Comment] No more neglect of helminths and HIV
Helminths are parasitic worms that disproportionately affect the world’s poorest people and cause chronic disease in a quarter of the global population.1 Helminth infections, such as lymphatic filariasis, schistosomiasis, and intestinal nematodiases, are classified by WHO as neglected tropical diseases.2 For years, these neglected tropical diseases have received minimal global attention and less than 1% of global research funding.3
Impact of the Australian National Cervical Screening Program in women of different ages
The known Following the 1991 introduction of the Australian National Cervical Screening Program, the incidence of cervical cancer declined, but trends for histological types in different age groups have not been reported.
The new Squamous cell cancer rates in women aged 25 years or more fell by more than 50%, but have now plateaued among women aged 25–69 years. Screening has had little impact on adenocarcinoma rates in any age group, and there was no decline in cervical cancer rates for 20–24-year-old women.
The implications Our findings support the planned 2017 transition to HPV-based screening starting at age 25, which may also reduce adenocarcinoma incidence.
The National Cervical Screening Program (NCSP) has been very successful in reducing the overall burden of cervical cancer in Australia by facilitating the detection and treatment of pre-cancerous lesions.1 However, in response to new evidence about the optimal age range for screening, new technologies, and the implementation of a successful national human papillomavirus (HPV) vaccination program, a major review of national cervical screening policy (the “renewal”) was recently undertaken.2 Recommended changes to the NCSP include a change from cytology-based screening every 2 years to primary HPV testing every 5 years (including partial HPV genotyping and the referral of HPV 16/18-positive women to colposcopy) and raising the age for starting screening from 18–20 years to 25 years.3,4
Concerns have been expressed about the safety of raising the screening age,5 although the change is consistent with international guidelines6 and with evidence that screening is of limited effectiveness in women under 25 years of age.7 It should also be noted that this change to the starting age is being undertaken in the context of high HPV vaccination coverage in young women in Australia, and of observed reductions in the rates of both high grade cervical abnormalities and of vaccine-included type infections (ie, infections with HPV 6, 11, 16 and 18), including in women who are potentially at higher risk.8–11
Earlier studies have examined how overall rates of cervical cancer have changed in Australia since the introduction of the NCSP,12 but no Australian study has analysed the effect by age group and histological subtype of cancer. Routine screening reports include incidence data classified according to either age or histological type, but not both, and do not include statistical analyses of trends.1 The aim of our study was to examine changes in the incidence of cervical cancer in Australia since the introduction of the current NCSP, taking both age and histological subtype of cervical cancer into account, in order to characterise the impact of the current program before the proposed changes to the NCSP are introduced.
Methods
Data sources
National cervical cancer incidence data for the period 1982–2010 were obtained from the Australian Institute of Health and Welfare. Age-specific rates were calculated, using population estimates from the Australian Bureau of Statistics.13 Three-year average rates were calculated for cervical cancer overall and separately for histological subtypes. The main analyses focused on squamous cell carcinoma (SCC) and adenocarcinoma, but trends for rarer subtypes (adenosquamous and other cancers) were also explored.
Statistical analysis
Standardised rate ratios (SRRs) compared the incidence in each overlapping 3-year period after the introduction of the NCSP with the 3-year average immediately preceding its inception (1988–1990). SRRs and 95% confidence intervals (CIs) were calculated by standard methods14 across all ages, for the target screening group (20–69-year-old women), and for the age groups 25–49, 50–69 and ≥ 70 years. Incidence rate ratios were calculated for the 20–24 years age group (as a single [non-composite] age group it could not be standardised). Joinpoint regression was undertaken to assess whether trends had been consistent over time and to estimate the annual percentage change in incidence. Joinpoint analysis fits the simplest trend model (fewest changes in trends) consistent with the data. To avoid overfitting, we restricted analyses to a maximum of two joinpoints (three trends) across the study period, with the a priori hypothesis that rates declined after the beginning of the NCSP, but allowing for the possibility that this decline had slowed during the second decade of the program, as suggested by visual inspection of the overall rates.
Statistical analysis was performed in SAS 9.3 (SAS Institute) and Joinpoint 4.2.0.2 (Surveillance Research Program, National Cancer Institute [USA]).
Ethics approval
Ethics approval was not required for the study, as only aggregated data were analysed.
Results
During 1982–2010, 26 236 cases of cervical cancer were registered in Australia (SCC, 18 626; adenocarcinoma, 4460; adenosquamous, 1080; other types, 2070). Since 1988–1990, the incidence of cervical cancer has declined, both overall and in all age groups examined, except for women aged 20–24 years. The reductions in incidence between 1988–1990 and 2008–2010 were primarily driven by declines in the rates of SCC (by 50%, 61% and 57% in women aged 25–49, 50–69 and 70 years or more respectively) (Box 1).
Joinpoint analysis indicated that the reduction in the incidence of SCC in women aged 25–49 years occurred mostly during the period 1990–2002 (Box 2, Box 3), without significant change outside this period. For women aged 50–69 years, SCC incidence was dropping prior to the introduction of the NCSP, with a stronger decline between 1994 and 2004, but without change from 2005 (Box 2, Box 3). The change from a decline to a plateau in the incidence of SCC around 2002–2004 was statistically significant for both age groups (P < 0.001). For women aged 70 years or more, the incidence of SCC declined before the introduction of the NCSP, but more rapidly from 1995 (Box 2, Box 3). For women aged 50–69 or 70 years or more, SCC incidence was thus dropping before the NCSP, but the subsequent rates of decrease were greater; these changes in trend were statistically significant (P < 0.001). There were no significant trends in SCC incidence in women aged 20–24 years before the inception of the NCSP, although point estimates suggest an increase until 1986, followed by a decline from 1987 to 1992, then a small, non-significant increase in SCC incidence from 1993. There were similarly no statistically significant trends in the overall incidence of cervical cancer in women aged 20–24 years before or after the start of the NCSP.
The incidence of adenocarcinoma across all ages was 18% lower in 2008–2010 than during 1988–1990. The difference was statistically significant for women aged 25–49 years, but not for other age groups (Box 1). However, there was no consistent downward trend for any age group (Box 4).
Rates of adenosquamous cancer were low, but the relative reduction and timing of the change in its incidence since 1988–1990 were similar to those for SCC in women aged 25–49 and 50–69 years (Appendix, Figures 1 and 2); case numbers among women aged 20–24 years were too small for analysis. The rate of other cervical cancer types appeared to decline across the entire period 1982–2010, without any change that could be related to the beginning of the NCSP, although absolute rates were small and the decline was not significant for women aged 20–24 or 70 years or more (Appendix, Figure 1 and Table 1). Prior to the start of the NCSP, other cervical cancers comprised a larger proportion of cervical cancers in women aged 20–24 years than for other age groups, although their incidence was still very low; they are now extremely rare in this age group (Appendix, Tables 2 and 3).
Discussion
Our analysis confirmed that SCC and overall cervical cancer rates have declined dramatically in women aged 25 years and over since the inception of the NCSP in Australia, but neither has declined in women aged 20–24 years. The overall decline among women aged 70 years or more, who are outside the target age range for screening, was understandably delayed compared with those for women aged 25–49 and 50–69 years, but the overall reduction is now comparable with that in the two younger age groups. While some women continue to be screened after age 69, their number is much smaller than for women in the target age range,1 and screening seems unlikely to explain a reduction in incidence of the magnitude measured. This suggests that the benefits of screening have extended beyond the screening end age in Australia, consistent with case–control data from England and Wales.15
Trends in the incidence of adenocarcinoma since the introduction of organised screening have been less uniform, consistent with findings in other settings of a limited impact of cytology-based cervical screening on adenocarcinoma rates.16,17 This difference has been attributed to the facts that cells from precursor lesions in the endocervical canal are more difficult to sample, and that glandular cells are more difficult to interpret than squamous cells.1
A reduction in the incidence of types of cervical cancer other than SCC was also observed, but appears unconnected with the NCSP, as it occurred throughout the entire study period. It could reflect improvements in clinical follow-up that may have led to improved identification of endometrial cancer that might previously have been misclassified as cervical cancer.
Our findings are timely, given the renewal of the NCSP and the changes in screening policy that will take effect in 2017. After considering the balance of benefits and harms, as recommended by the Australian Screening Framework,18 the Medical Services Advisory Committee (MSAC) recommended that from 2017 women under 25 years of age no longer be screened.3 Our findings support this recommendation: although women aged 20–24 years have been included in the NCSP for more than 20 years, there has been no significant impact on the incidence of either SCC or of cervical cancer overall in this age group. These women also now have a substantially lower risk of cervical cancer because of HPV vaccination. Women under 25 years of age in 2017 will have been offered HPV vaccination at school before they were 15 years old, and the three-dose vaccine uptake rate in these women exceeds 70%.19 The prevalence of vaccine-included HPV types is already very low among women under 25, even among unvaccinated women and woman at potentially higher risk.9–11 High grade cytological abnormality rates have fallen in this age group,8 even though uptake of vaccination in cohorts where this reduction has already been reported was less than 70%, and its efficacy may have been reduced by prior exposure to the virus because these women were vaccinated as young women or older adolescents. Both direct protection and indirect protection for unvaccinated women via herd immunity is likely to be even greater in younger birth cohorts:9 direct protection because females vaccinated as younger adolescents have higher vaccination coverage and lower rates of HPV exposure prior to vaccination; indirect protection because more of the population has since been vaccinated, and because boys are now offered vaccination.20
While screening women under 25 years of age does not appear to substantially affect the incidence of cervical cancer among 20–24-year-old women, it is possible that it might reduce cancer rates among women aged 25–29 years by detecting and treating pre-cancerous lesions before the age of 25. This possibility could not be directly assessed in our study, but data from a UK case–control study suggest that being screened between the ages of 22 and 24 years does not reduce the risk of cancer for women aged 25–29 years.7 An important change to the NCSP from 2017 is that women will receive explicit invitations to attend screening, receiving the first close to their 25th birthday. A switch from a reminder-based to an invitation-based program was a key recommendation of the MSAC,3 and modelling indicates this change will have an important impact on the effectiveness of the program in young women, and on the program overall.21 A wide range of program designs were modelled, and it was estimated that inviting women at age 25 would reduce cervical cancer incidence across all ages by about 2% compared with an otherwise identical program without invitations (in which case screening would probably commence more gradually between the ages of 25 and 29 years).21
An alternative explanation for our finding that the incidence of SCC and of cervical cancer overall in women aged 20–24 years did not decrease after the start of the NCSP is that the impact of the program has been limited by falling screening participation in this age group. However, while their 2-year participation rates have fallen since reporting began (1996–1997), there have been similar falls in participation rates for women aged 25–29 and 30–34 years, among whom cancer rates have declined.22 Additionally, participation by women aged 20–24 years has mainly fallen since 2006–2007, and this would be unlikely to have affected our findings, because the reductions in cancer incidence in other age groups predominantly occurred within the first 10–15 years of the organised screening program, with little change in rates in recent years.
Another possibility is that screening women aged 20–24 years has suppressed a rise in cervical cancer that would otherwise have followed a hypothetical increase in risk behaviour among young women (eg, first intercourse or more sexual partners at a younger age). The results of sexual behaviour surveys are inconclusive as to whether such an increase has occurred, and therefore about whether it would affect our findings. Data from a national population-based survey of sexual behaviour indicate that the median age at first vaginal intercourse was the same for women aged 20–24 years before and immediately after the start of the NCSP (ie, women born 1965–1974) as it was for women who were 20–24 years old during the remainder of period covered by our analysis (ie, women born 1975–1990).23 However, this study also reported a difference between these cohorts in the proportion of women who reported first intercourse before the age of 16 years (a rise from 12.7% to 18.2%).23 There are no data on the number of sexual partners before the age of 20 years that would allow a comparison of these cohorts. Although we cannot exclude an increase in risk behaviour, it seems unlikely that it would fully explain the observed stable incidence of cervical cancer among 20–24-year-old women because, given the magnitude of the reductions in rates in other age groups, it presupposes that a major increase in risk behaviour coincided with the period of the NCSP.
A limitation of our analysis is that cervical cancer incidence rates reported here were not adjusted for hysterectomy rates, as data for this factor were not available for the entire study period. This limitation is common to all routine reports on cervical cancer incidence in Australia,8 but it means that the denominator does not perfectly reflect the true population at risk of cervical cancer. We may therefore have underestimated the incidence of cervical cancer in older women. However, this limitation would not affect our findings for women aged 20–24 years, and would probably have only a small impact on our findings for those aged 25–49 years. Reductions in rates for older women may have been overestimated if part of the drop was attributable to rates of hysterectomy increasing since the mid-1990s, but survey data suggest that they did not.24,25 Further, as the overall reductions in cancer incidence were substantial, they are unlikely to be fully explained by changes in hysterectomy rates.
The current NCSP has been highly successful in reducing the incidence of squamous cervical cancer in Australia, by at least 50% in women aged 25 years or more. However, its effectiveness has been limited among women under 25, and in reducing adenocarcinoma rates. Further, as participation in screening has plateaued (and is falling in some age groups), cervical cancer incidence also appears to have plateaued, if at a lower level than before the program. The National HPV Vaccination Program and the renewed NCSP have the potential to mitigate these limitations. HPV vaccination will provide protection for younger women, while both HPV vaccination and HPV-based screening are expected to reduce adenocarcinoma rates.26 It is estimated that the renewed NCSP will reduce cervical cancer incidence and mortality by at least a further 20%,3,4,21 assuming that active invitations and recalls are effective in achieving high participation rates. In the longer term, the combination of HPV vaccination and the renewed NCSP is predicted to reduce cervical cancer incidence by about 70% below what would have been expected without program change and vaccination,4 and will therefore further reduce the burden of cervical cancer among women in Australia.
Box 1 –
Cervical cancer incidence (per 100 000 women) and standardised rate ratios (SRRs) comparing the 3-year average incidence of cervical cancer during 2008–2010 with the 3-year average incidence immediately prior to inception of the National Cervical Screening Program (1988–1990), by histological cancer type and age group
|
|
Squamous cell carcinoma |
Adenocarcinoma |
All cervical cancer |
||||||||||||
|
1988–1990 |
2008–2010 |
SRR (95% CI) |
1988–1990 |
2008–2010 |
SRR (95% CI) |
1988–1990 |
2008–2010 |
SRR (95% CI) |
|||||||
|
|
|||||||||||||||
|
All ages |
9.9 |
4.5 |
0.46 (0.43–0.49) |
2.0 |
1.6 |
0.82 (0.72–0.93) |
13.5 |
7.0 |
0.51 (0.49–0.54) |
||||||
|
20–69 years |
13.2 |
6.1 |
0.46 (0.43–0.50) |
2.7 |
2.3 |
0.84 (0.73–0.96) |
18.0 |
9.3 |
0.52 (0.49–0.55) |
||||||
|
20–24 years* |
1.5 |
1.3 |
0.91 (0.55–1.51) |
0.4 |
0.4 |
0.91 (0.35–2.40) |
2.6 |
1.8 |
0.70 (0.46–1.05) |
||||||
|
25–49 years† |
13.4 |
6.7 |
0.50 (0.46–0.55) |
3.2 |
2.7 |
0.83 (0.70–0.97) |
18.9 |
10.3 |
0.55 (0.51–0.59) |
||||||
|
50–69 years† |
16.7 |
6.6 |
0.39 (0.35–0.45) |
2.6 |
2.2 |
0.86 (0.67–1.10) |
21.7 |
10.0 |
0.46 (0.42–0.51) |
||||||
|
≥ 70 years† |
16.8 |
7.2 |
0.43 (0.36–0.51) |
2.8 |
2.0 |
0.71 (0.50–1.02) |
22.7 |
11.4 |
0.50 (0.43–0.58) |
||||||
|
|
|||||||||||||||
|
* Results presented as age-specific incidence rates and age-specific incidence rate ratios. † Results presented as age-standardised incidence rates and standardised rate ratios, using the Australian 2001 Standard Population.13 |
|||||||||||||||
Box 2 –
Three-year average cervical cancer incidence (with 95% CIs), by age and histological type, 1982–2010
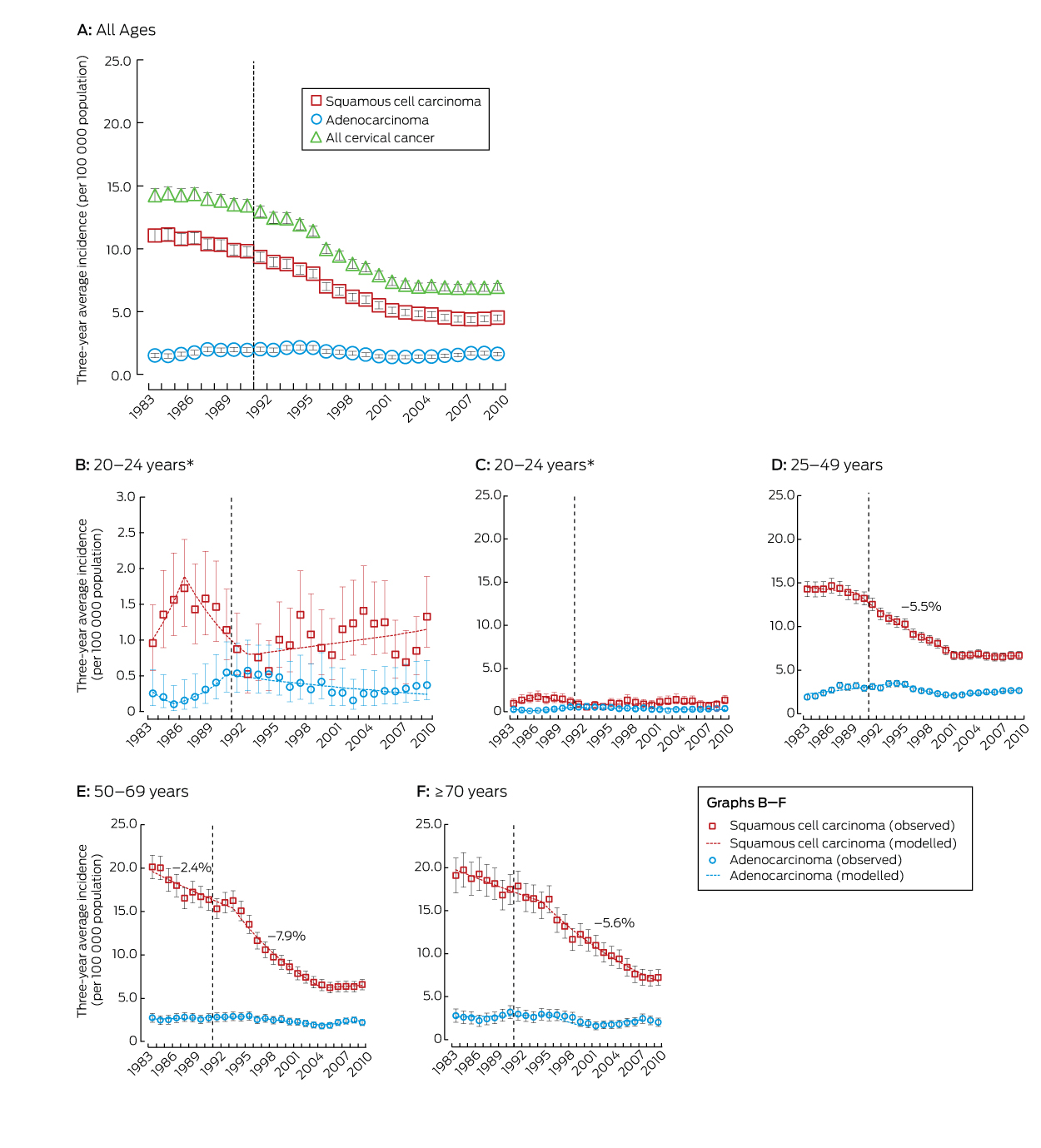
The dashed line represents the start of the National Cervical Screening Program in Australia. Rates for all ages and for the age groups 25–49, 50–69 and ≥ 70 years were standardised, using the Australian 2001 Standard Population.13 The annual percentage changes in incidence rates are included for periods when the change was significant at P < 0.05. * Results for the age group 20–24 years are depicted twice; the vertical axis scale in panel C is the same as for the other age groups, to assist comparison, while panel B uses a compressed vertical axis for clearer display.
Box 3 –
Annual percentage change in the 3-year average incidence of squamous cell carcinoma, by age
|
Age group/period |
Annual change in incidence (95% CI) |
P |
|||||||||||||
|
|
|||||||||||||||
|
20–24 years |
|
|
|||||||||||||
|
1983–1986 |
23.1% (–14.2% to 76.6%) |
0.24 |
|||||||||||||
|
1987–1992 |
–13.4% (–26.3% to 1.7%) |
0.08 |
|||||||||||||
|
1993–2009 |
2.2% (–0.4% to 4.8%) |
0.09 |
|||||||||||||
|
25–49 years |
|
|
|||||||||||||
|
1983–1989 |
–0.8% (–1.7% to 0.2%) |
0.12 |
|||||||||||||
|
1990–2002 |
–5.5% (–5.9% to –5.2%) |
< 0.001 |
|||||||||||||
|
2003–2009 |
–0.01% (–1.0% to 0.9%) |
0.99 |
|||||||||||||
|
50–69 years |
|
|
|||||||||||||
|
1983–1993 |
–2.4% (–3.1% to –1.7%) |
< 0.001 |
|||||||||||||
|
1994–2004 |
–7.9% (–8.7% to –7.1%) |
< 0.001 |
|||||||||||||
|
2005–2009 |
0.9% (–1.8% to 3.6%) |
0.5 |
|||||||||||||
|
≥ 70 years |
|
|
|||||||||||||
|
1983–1994 |
–1.9% (–2.6% to –1.1%) |
< 0.001 |
|||||||||||||
|
1995–2009 |
–5.6% (–6.1% to –5.1%) |
< 0.001 |
|||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 4 –
Incidence rate ratios (with 95% CIs) comparing the 3-year average cervical incidence with the 3-year average immediately before the start of the National Cervical Screening Program (1988–1990), by histological type and age
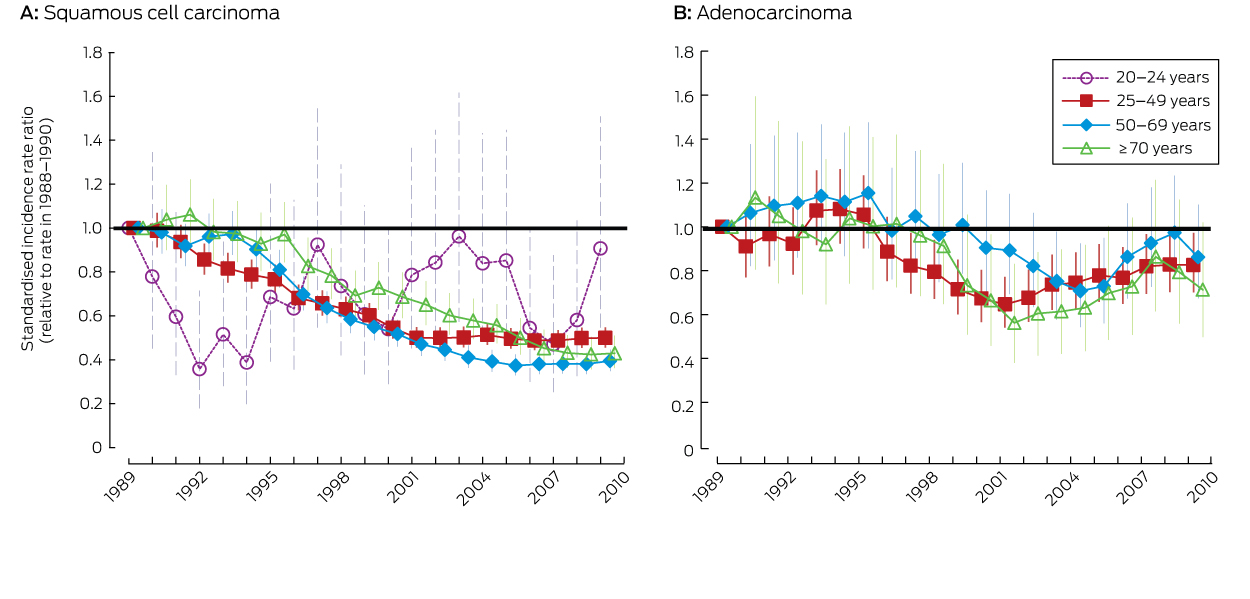
Data for the age groups 25–49, 50–69 and ≥ 70 years are presented as standardised rate ratios, using the Australian 2001 Standard Population.13 Data for the 20–24 years age group are presented as age-specific incidence rate ratios.
Women’s health: local and global matters of great significance
A life cycle approach is important, as is acknowledging the importance of socio-cultural and lifestyle factors
Women’s health, in its broadest sense, encompasses all aspects of their health and wellbeing. From this perspective, this issue of the MJA includes a wide selection of articles covering key issues in women’s health, both locally and globally. The topics covered are diverse, and include pregnancy and reproductive health, as well as health and wellbeing at various stages of a woman’s life cycle. Taking a life course perspective of women’s health clarifies links between their socio-cultural background, reproductive health, lifestyle, and chronic disease risk.1 Significant events across the lifespan, including birthweight and age of menarche, have been identified as likely markers of cardiovascular disease risk,2 pre-menopausal breast cancer risk,3 and diabetes4 in women.
Most women in high and middle income countries will come into contact with health systems and health professionals while they are pregnant, but in Australia there is a confusing plethora of models of care. In some models the care is fragmented, as women move between primary and secondary care, private and public services, and medical and midwifery providers. Outcomes of pregnancy are important indicators of health for women and their families, so it is essential that women have access to a model of care that provides them with the best possible outcomes in every respect.
The narrative review by Homer5 examines the evidence in favour of continuity of care models in which a midwife is the primary maternity caregiver. The evidence, much of which is from Australia, is very clearly in favour of such models. Women report high levels of satisfaction with the midwife’s holistic approach to care during pregnancy and the postnatal period; the maternal and perinatal outcomes are the same as for other medical models, and are achieved with less intervention and at lower cost. The considerable high level evidence from randomised clinical trials now forms the basis of guidelines that advocate this approach for low or normal risk women. Why is it then so difficult for many women to choose this evidence-based model of midwifery care during pregnancy? Only a minority of women can access this form of care, and maternity hospitals appear reluctant to recognise the evidence. Inter-professional rivalries, lack of collaborative leadership models, and inaccurate citing of evidence all block further development, and the translation of the available evidence into practice is held up. As Homer remarks, it really needs to be asked whether it is ethical to deny women access to a model of care that is so strongly supported by the evidence.
Further assessment of how effectively these models work for women with higher risks during pregnancy is required, and one of the greatest challenges is ensuring that all health professionals involved in maternity care work collaboratively to achieve the best outcomes. It makes sense that women at higher psycho-social risk would benefit from greater continuity. Whether or not such models lead to better medium and long term psychological and emotional outcomes for all women needs to be determined, but there is potential for significant benefits for the entire family if the model enhances their wellbeing in the postnatal period and beyond. The likelihood that this outcome of pregnancy will impact on women later in life should be obvious to all.
In their later years, non-communicable diseases (NCDs) pose one of the greatest threats to women’s health globally. NCDs such as cardiovascular disease, cancer, diabetes, and chronic respiratory diseases currently account for around 18 million deaths in women annually, and it is estimated that this will rise by 17% over the next decade.6 The perspective article on global women’s health by Davidson and colleagues7 identifies that much of the increased risk has been attributed to socio-cultural factors, although lifestyle factors, such as unhealthy diet, alcohol consumption and smoking, physical inactivity, and obesity, also play pivotal roles.6 Davidson and colleagues argue that the ramifications of the burden of NCDs for women, their families and the global community is significant, and will lead to escalating health care costs, lost productivity, and adverse social and economic outcomes for families.7
Importantly, the perspective article by Teede and colleagues8 explains how biological differences, gender roles, and social marginalisation affect women, and mean that their risk behaviours are not the same as those of men, with consequences for the success of health-related interventions. From this perspective, more targeted health programs for women and health models of care are likely to better promote the wellbeing of women generally, as well as during pregnancy. This approach should help reduce the impact of modifiable risk factors and the burden of chronic disease, and enhance the development of comprehensive evidence-based policy and practice that improve women’s health.
There is increasing evidence that women’s health needs, both locally and globally, are best served by interventions tailored to their specific needs, acknowledging the links between socio-cultural background, reproductive health, lifestyle and chronic disease risk. Improving health outcomes for women, during pregnancy and at other stages of their life cycle, requires health service providers to recognise this, and to use the evidence to inform their provision of care that is both effective and acceptable to women.
[Articles] Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015
Declines in some key environmental risks have contributed to declines in critical infectious diseases. Some risks appear to be invariant to SDI. Increasing risks, including high BMI, high fasting plasma glucose, drug use, and some occupational exposures, contribute to rising burden from some conditions, but also provide opportunities for intervention. Some highly preventable risks, such as smoking, remain major causes of attributable DALYs, even as exposure is declining. Public policy makers need to pay attention to the risks that are increasingly major contributors to global burden.
[Articles] Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015
Ageing of the world’s population is increasing the number of people living with sequelae of diseases and injuries. Shifts in the epidemiological profile driven by socioeconomic change also contribute to the continued increase in years lived with disability (YLDs) as well as the rate of increase in YLDs. Despite limitations imposed by gaps in data availability and the variable quality of the data available, the standardised and comprehensive approach of the GBD study provides opportunities to examine broad trends, compare those trends between countries or subnational geographies, benchmark against locations at similar stages of development, and gauge the strength or weakness of the estimates available.
[Editorial] GBD 2015: from big data to meaningful change
The Global Burden of Diseases, Injuries, and Risk Factors Study 2015 (GBD 2015) brings together the most recent epidemiological data according to year, age, and sex from 195 countries and territories. In a continuation of the partnership between The Lancet and the Institute for Health Metrics and Evaluation (IHME), this year marks the first of an annual commitment to publish the four capstone GBD papers—on global mortality, years lived with disability, disability-adjusted life-years, and risk factors—in a single issue.

 more_vert
more_vert