Neurologists are often perceived as diagnosticians with little in the way of treatments to benefit their patients. However, this view is being gradually dispelled. In no area of neurology is this occurring more rapidly than in neuroimmunology, where recognition of constellations of clinical signs can lead to prompt and effective treatment, often preventing serious sequelae.
Preference: Immunology and Allergy
411
The Australian Lupus Registry and Biobank: a timely initiative
A collaborative effort to provide real world evidence for therapies for patients with lupus
Systemic lupus erythematosus (SLE) is a complex autoimmune disease with diverse clinical manifestations, which places an unacceptable level of burden on affected patients. Australian data on lupus are scarce, with figures suggesting a prevalence of SLE that ranges from 19 per 100 000 in people of European ancestry to 92 per 100 000 in Indigenous Australians,1 similar to other chronic diseases such as hepatitis C.2 Survival rates for SLE patients in the 1950s were as low as 50% at 5 years. With improvements in the treatment of renal disease and infection, survival rates in most studies improved to around 90% at 10 years by the 1990s. However, it is still a sobering thought that SLE, which typically presents in women in their second or third decade of life, confers a 1 in 10 chance of dying before the age of 40.3 Damage accumulation, long term medication side effects (particularly steroids side effects), fatigue and uncertainty profoundly affect quality of life.4 Fundamental data regarding age, geographic and ethnic distribution; natural history of the disease; currently used treatments; and unmet needs of patients in Australia have not been well defined.
Since it is a relatively rare and heterogeneous disease, longitudinal registry studies play an essential role in improving our understanding of SLE. Registry studies are ideally suited to capturing real world data on a large number of subjects, giving insights into disease course and treatment practices. Moreover, they may serve as a platform to inform planning of randomised controlled trials (RCTs) and subcohort studies. In contrast to RCTs, they typically have broader inclusion criteria and allow for long term follow-up. Registry findings can complement RCT results, as demonstrated by the increased risk of tuberculosis associated with the use of tumour necrosis factor inhibitors in patients with rheumatoid arthritis, which was not identified in clinical trials, but rather revealed and quantified through registry studies.5 Despite the inherent limitations, a great deal can be learnt from single-centre SLE registries, such as the seminal 1974 observation in the Toronto lupus cohort of a bimodal mortality pattern, with early deaths due to active disease and infection, and later deaths due to premature cardiovascular disease.6 However, much progress has been made in past 40 years, with large multicentre cohorts from predominantly Europe and North America contributing to our understanding of the disease, particularly with reference to real world epidemiology, clinical features, natural history and long term outcomes.7
A national registry based in Australia may be a late starter, but it has the potential to be a world leader, with carefully collected data that provide assessment of visit-to-visit disease activity and of medication exposure. It also enables the conduct of studies specific to the Australian health care settings and the demographics of a multicultural Australian population. The Australian Lupus Registry and Biobank (ALRB) was created in 2012, with seed funding from MOVE Muscle, Bone and Joint Health (formerly Arthritis and Osteoporosis Victoria) and contributions from the industry in the form of unrestricted grants.
The ALRB is an online platform that enables the longitudinal collection of systematic and comprehensive data. One of the first studies using the registry aimed at understanding the disease characteristics and treatment patterns in Australia. Using the framework provided by the registry, we have also undertaken a study to validate a consensus-based treatment target to determine whether this can be a data-driven treatment endpoint associated with better patient outcomes.8 The effects of long term use of immunosuppressive medication in SLE patients are not well understood, and data from the registry may give us a better understanding of the incidence of adverse effects and benefits, such as reduction in flares and accumulated damage over time. In addition, biomarker studies examining the interferon-α gene signature and disease manifestations, response to treatment, vitamin D status and disease manifestations, and patient-reported quality of life are in the planning stages.9–12
The registry also collects patient-reported outcomes, such as Short Form 36 and multidimensional health assessment questionnaires. Patients’ self-reported data complement physician-reported data in the ALRB to capture the breadth of experiences of patients with SLE in Australia and provide a meaningful assessment of the disease burden and treatment shortfalls.
There are now ten institutions recruiting patients with SLE to the ALRB across Victoria, New South Wales, South Australia and Western Australia, with the common goal of improving treatment and outcomes for people with SLE. The expansion of the ALRB to involve more practices across Australia may be easier if the platform imports from existing electronic medical records or health systems (administrative, laboratory or radiological) to avoid duplicated data entry — provided data fields are matched according to a stringent data dictionary. Periodic auditing, involving cross-checking data with source records, will be done by principal investigators at each site and will be reported to the ALRB Management Committee to ensure data completeness and accuracy. This committee may also request the de-identified source documentation for quality assurance purposes.
One of the key strengths of the ALRB rests in its ability to examine a variety of health care outputs over time. At present, in the complex Australian health care system, it is difficult to examine the different components of health care use; therefore, the true economic costs for a disease such as SLE are often grossly underestimated. The ALRB will allow the tracking of health care uses related to the care of the SLE in Australia and will provide data for benchmarking. With the rising costs of health care and a limited health budget, it is paramount that data are available to study the cost effectiveness of various management strategies. Health care use, based on annual patient self-reporting of hospitalisations, investigations and other health complications, may form the basis to derive cost. The ALRB information may help measure the health consequences of different health care interventions.
The overarching principle of the ALRB is to foster collaborative research and, with the same purpose, the simultaneous development of the Asia–Pacific Lupus Collaboration (www.asiapacificlupus.com) brings together researchers from Australia, China, Dubai, Hong Kong, Indonesia, Japan, Malaysia, Philippines, Singapore, Taiwan and Thailand. More than 2000 patients with lupus across the Asia–Pacific region have been recruited in the Lupus Low Disease Activity State study to validate a treatment target.8 This type of research is consistent with the Australian Research Council strategy to encourage international collaboration — especially where it has been led by the Australian site (www.arc.gov.au/international-collaboration).
Finally, the parallel development of a biobanking system to complement clinical data from the ALRB means that more questions into aetiology and novel biomarkers can be answered. The fostering of closer links between basic science researchers and clinicians is the foundation of good translational research. Linking clinical phenotype to genetic polymorphisms and novel laboratory parameters has been valuable in understanding pathogenesis and prognosis, and in predicting SLE manifestations and response to treatment in such a heterogeneous disease.13
The ALRB is still in its infancy and will require significant inputs from various funding sources to continue its growth. We expect that, as the registry grows, it will serve as a valuable resource for clinicians, scientists, epidemiologists, patient advocacy groups, industry and government to provide real world evidence of clinical effectiveness of existing or new therapies and management strategies in patients with SLE in Australia.
Australian law needs a refresher on the science of HIV transmission
Being diagnosed with HIV is a confronting experience.
However the stigma associated with HIV infection – a hangover from its social and medical history – is responsible for an exaggerated perception of transmission risk through sex, and the harms of living with HIV infection.
In our consensus statement published this week in the Medical Journal of Australia, we detail the latest evidence on HIV transmission risk and recent advances in HIV prevention and treatment.
We propose that legal cases relating to HIV transmission should be considered in light of such evidence, and that alternatives to prosecution such as the public health management approach are often appropriate.
HIV infection no longer a death sentence
There have been many advances in HIV diagnosis, prevention and treatment since the identification of the first AIDS cases in the early 1980s.
In the initial days of the AIDS epidemic, patients would, after a number of years, develop serious infections and other illnesses due to their immune deficiency, usually resulting in death. When the first treatments became available, they bought time but often at the cost of serious medication side-effects, and complicated treatment regimens involving many tablets each day.
While it remains a serious infection, HIV is now a disease that can be effectively managed through medical treatment, regular health monitoring and healthy lifestyle. For many people with HIV, treatment involves taking only a single pill each day. Those taking antiviral therapy can expect to live a normal life, in good health, with a life expectancy similar to their HIV-negative counterparts.
These great improvements, familiar to those working in health, are not as well understood in the legal sector.
Prosecutions for HIV infection
Unlike other diseases, HIV has a long and uneasy relationship with criminal law. In the early years of the epidemic, the stigma around HIV, the fact that it was almost always fatal, and unfounded fears about its potential use as a weapon led to the criminalisation of HIV transmission and exposure.
Since 1991, there have been more than 38 criminal prosecutions for HIV transmission or exposure during sex in Australia. Despite the significant improvements in health and longevity of people living with HIV, the rate of criminal prosecutions has not decreased.
The courts have shown an understanding of the effectiveness of condoms: no one who has used condoms has been convicted. However, people continue to be prosecuted, including for “exposing” others to the risk of HIV infection, even in the absence of actual transmission. This occurs despite the relatively low per-act risk of HIV transmission and the fact that for most people the harms of HIV infection are far less serious than they once were.
New approaches to limit HIV transmission
HIV is actually difficult to transmit. Sexual transmission occurs during only about 1% (or less) of penetrative sexual encounters, even when a condom is not used and the HIV-positive person is not on treatment.
HIV prevention messaging in the early days of the epidemic focused on sexual abstinence and condom use. However, prevention messaging is now more nuanced and has expanded to include new ways of reducing HIV transmission risk. A not insignificant number of people at risk of HIV infection choose to have sex without using a condom, which is why developing alternative methods of HIV prevention have been prioritised in recent years. Such research has delivered game-changing results: “treatment as prevention” and “pre-exposure prophylaxis”.
Treatment as prevention refers to the greatly reduced risk of HIV transmission as a result of HIV-positive people taking antiviral treatment, which suppresses replication of the virus in the infected person’s body. When a person with HIV has a very low viral load (unmeasurable levels of HIV in the blood), the risk of sexual transmission becomes very low. In fact, there has never been a documented case of HIV transmission from a person with an undetectable viral load.
Pre-exposure prophylaxis describes the use of antiviral medication by an HIV-negative person as a way of preventing HIV infection. Pre-exposure prophylaxis is a very effective means of preventing HIV transmission, with only isolated cases of transmission identified among people applying this approach. This groundbreaking new strategy is available in Australia via limited pilot programs, and is being evaluated for Pharmaceutical Benefits Scheme listing.
How the law treats people with HIV
Criminal laws relating to HIV transmission and exposure vary from state to state, but a common factor is that people with HIV are expected to take “reasonable precautions” to prevent transmission. Condom use has long been accepted as meeting this threshold.
The evidence now supports acceptance of treatment as prevention (for the positive partner) and/or pre-exposure prophylaxis (for the negative partner) as meeting the same standard. And the limited harms of HIV infection as a consequence of acts involving low to negligible risk of transmission mean HIV cases generally do not belong in criminal courts.
There is an alternative. All states and territories have health protocols for managing allegations of risky behaviour. This public health approach – involving education, case management and, where required, behavioural orders and isolation – is a much more effective way of protecting public health.
As researchers and clinicians, we are intimately aware of the impact an HIV diagnosis can have. We have all supported patients coming to terms with an HIV diagnosis; many of us having had the painful task of delivering that devastating news.
The criminal law has a role to play, particularly should there ever be a case where a person deliberately transmits HIV.
However, with the advances of recent years in both prevention and treatment, authorities need to be more familiar with latest scientific and medical evidence, and consider alternatives to prosecution such as the public health management approach.
Elizabeth Crock, Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine, co-authored the consensus statement.
![]() Mark Boyd, Professor, Chair of Medicine, University of Adelaide; Andrew Grulich, Professor and Program Head, UNSW Australia; David Cooper, Scientia Professor of Medicine and Director, Kirby Institute, UNSW Australia; David Nolan, Adjunct Professor, Institute for Immunology and Infectious Disease , Murdoch University; Levinia Crooks, Adjunct Associate Professor, La Trobe University; Michelle Giles, Associate Professor, Department of infectious diseases and Dept of Obstetrics and gynaecology, Monash University; Sharon Lewin, Director, The Peter Doherty Institute for Infection and Immunity, The University of Melbourne and Royal Melbourne Hospital and Consultant Physician, Department of Infectious Diseases, Alfred Hospital and Monash University, The Peter Doherty Institute for Infection and Immunity, and Trent Yarwood, Infectious Diseases Physician, Senior Lecturer, James Cook University and, The University of Queensland
Mark Boyd, Professor, Chair of Medicine, University of Adelaide; Andrew Grulich, Professor and Program Head, UNSW Australia; David Cooper, Scientia Professor of Medicine and Director, Kirby Institute, UNSW Australia; David Nolan, Adjunct Professor, Institute for Immunology and Infectious Disease , Murdoch University; Levinia Crooks, Adjunct Associate Professor, La Trobe University; Michelle Giles, Associate Professor, Department of infectious diseases and Dept of Obstetrics and gynaecology, Monash University; Sharon Lewin, Director, The Peter Doherty Institute for Infection and Immunity, The University of Melbourne and Royal Melbourne Hospital and Consultant Physician, Department of Infectious Diseases, Alfred Hospital and Monash University, The Peter Doherty Institute for Infection and Immunity, and Trent Yarwood, Infectious Diseases Physician, Senior Lecturer, James Cook University and, The University of Queensland
This article was originally published on The Conversation. Read the original article.
Latest news
Management of adverse events related to new cancer immunotherapy (immune checkpoint inhibitors)
Important therapeutic advances have been made in the field of cancer immunotherapy in recent years. In particular, immune checkpoint inhibitors (ICIs) have revolutionised the treatment landscape of advanced cancer over the past 5 years, with 15–20% of patients achieving long term disease control beyond 5 years.1 ICIs are monoclonal antibodies which block cell surface molecules involved in the regulation of T cell activation, including cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1) and its ligand (PD-L1). In normal homoeostasis, these inhibitory molecules are involved in preventing excessive inflammation and autoimmunity.2 However, in the tumour microenvironment, these molecules are overexpressed and promote immune tolerance rather than tumour destruction.3 Blockade of these molecules can restore the appropriate anti-tumour response and potentially improve patient survival (Box 1). Other inhibitory and activating molecules are also involved in balancing T cell regulation, some of which are undergoing further research as therapeutic targets.2–4
ICIs with Pharmaceutical Benefits Scheme funding for use in Australia currently include ipilimumab (an anti-CTLA-4 antibody), nivolumab and pembrolizumab (anti-PD-1 antibodies) for advanced malignant melanoma. Besides these, a number of other ICIs are undergoing evaluation in clinical trials across Australia and globally. These include durvalumab, atezolizumab and avelumab (anti-PD-L1 antibodies) and tremelimumab (an anti-CTLA-4 antibody). ICIs have been most utilised in the management of advanced melanoma, non-small cell lung cancer and metastatic renal cell carcinoma. However, they have shown some benefit in a range of other malignancies including Hodgkin lymphoma, gastric cancer and prostate cancer.5 Therefore, the number of patients receiving ICIs is likely to increase in the coming years.
Immune-related adverse events (irAEs) are a direct consequence of impaired self-tolerance from loss of T cell inhibition and altered immune regulation. They encompass a distinctive range of autoimmune toxicities, which differ significantly from the side effects of cytotoxic chemotherapy. Any organ can potentially be involved, with dermatologic, gastrointestinal, hepatic or endocrine toxicity occurring most frequently.5,6 The incidence of the most common irAEs is presented in Box 2.7–14
While prescription of ICIs may be limited to oncology specialists, knowledge of ICIs and their toxicities is relevant to a wide range of health care professionals who may encounter patients experiencing such complications.
This article describes the most common and clinically important irAEs that occur with ICIs and summarises the available evidence on management.
Literature search
A PubMed search was performed for articles published until 30 April 2016 using key terms “immune checkpoint inhibitor”, “CTLA-4”, “PD-1”, “PD-L1”, “ipilimumab”, “pembrolizumab” and “nivolumab”, and “immune-related adverse event”, “toxicity” and “side effects”. Product information for ipilimumab, pembrolizumab and nivolumab was accessed online through the manufacturers’ websites.
General management of irAEs
Most irAEs are generally manageable if identified and treated promptly. However, some patients can experience life-threatening toxicity leading to morbidity and mortality. The importance of patient education in the management of irAEs cannot be overemphasised. Detailed education and written information should be provided to all patients along with a medical alert card highlighting the name of the drug and who to contact in case of emergency. Patients should be advised to promptly report any potential toxicity as delay in the initiation of treatment of irAEs can lead to serious consequences. Patients should be managed at, or in close consultation with, centres familiar with the use of ICIs and treatment of irAEs with clearly defined management algorithms, especially to support junior medical staff who might not be familiar with these drugs. Specialist oncology nurses should be involved in patient education and maintain regular contact to monitor for side effects. Safety checklists should also be followed during routine medical oncology clinic reviews, bearing in mind the toxicity profile of these agents.
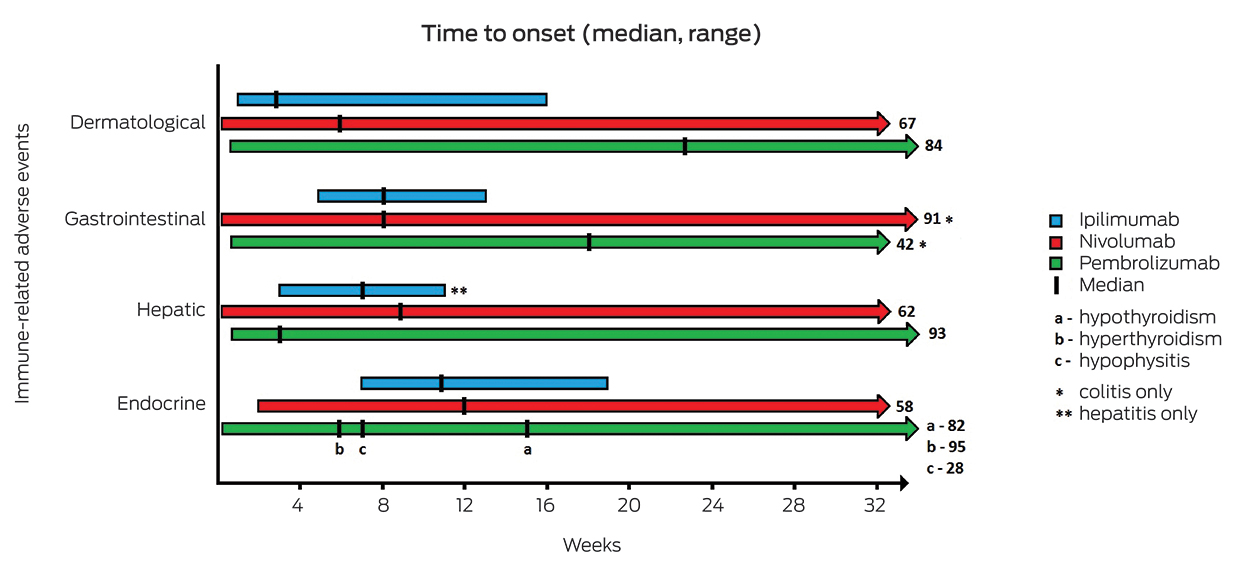
The time to onset of irAEs varies depending on the ICI and the organ affected (Box 3). With ipilimumab, most irAEs occur during the initial induction phase, although toxicity can develop even after treatment has been completed.5 Dermatological irAEs typically emerge first, followed by gastrointestinal, hepatic and then endocrine side effects.6,15,16 The median time to onset tends to be later with anti-PD-1 agents, and the range of time over which irAEs occur is much longer than for ipilimumab.17,18
Algorithms to guide management of common irAEs related to ipilimumab were developed by the manufacturer. These algorithms can be accessed online.19 They have been widely adopted and similar algorithms have been applied to other ICIs;17,20 however, no prospective trials have been performed to determine optimal management regimens. Some institutions, including the Fiona Stanley Hospital in Perth (Immune-related adverse events associated with use of ICIs: clinical guideline, unpublished internal document), have developed their own multidisciplinary guidelines to ensure a standardised approach to treatment of irAEs.21
Treatment depends on irAE type and severity, with the grading system outlined in Common Terminology Criteria for Adverse Events version 4.022 used to accurately and objectively gauge severity (Box 4).
Oral or intravenous (IV) corticosteroids are frequently used in the management of irAEs, depending upon the grade of toxicity. Most of the irAEs can be managed with early detection and prompt initiation of high dose steroids. Steroid tapering should be gradual over 2–4 weeks once patient symptoms are improving. Before resuming ICIs, irAE severity should return to grade 1 or have resolved entirely. Other immunosuppressive or immunomodulatory therapies may be indicated for severe or steroid-refractory cases. Prophylactic antibiotics to prevent opportunistic infections should be considered during immunosuppression, including prolonged systemic steroid therapy.5,21
ICIs are usually withheld while irAEs are treated, and permanently discontinued in severe cases. For mild or moderate irAEs, the decision to reintroduce ICIs requires careful consideration of the risks and benefits. The ongoing need for additional immunosuppression has been considered a contraindication to reintroducing ICIs.15,17,21 However, the actual risk of developing recurrence of irAEs in this situation is not known. It may be reasonable to consider further cautious use of ICIs if irAEs are controlled with low dose immunosuppression, depending on the balance of risks and benefits for individual patients.
Dermatological toxicity
Skin irAEs generally develop within a few weeks of commencing treatment, although delayed onset of rash has also been reported.23 Pruritus and rash are commonly reported with all ICIs (Box 2).24,25 The typical rash is maculopapular and mild, although severe cutaneous reactions such as Stevens–Johnson syndrome or toxic epidermal necrolysis, drug rash with eosinophilia and systemic symptoms, and pyoderma gangrenosum-like ulceration have been reported with ipilimumab.24,26 The management of rash is outlined in Box 5.
Vitiligo has been reported in patients treated for melanoma but not in other cancers.24,25 Vitiligo is considered to be potentially predictive of durable response to ICIs.27
Mild, localised pruritus usually responds to the combination of oral antihistamines and topical corticosteroids. Intense or widespread pruritus may require systemic corticosteroids (prednisolone 0.5–1 mg/kg/day). Mirtazapine or γ-aminobutyric acid agonists, such as gabapentin or pregabalin, have been suggested for intractable pruritus.24
Gastrointestinal toxicity
Diarrhoea occurs frequently with ICIs, with the highest incidence reported with combination therapy with ipilimumab and nivolumab (Box 2).8 Diarrhoea may occur with colitis, with signs and symptoms including blood or mucous in the stool and abdominal pain.5 Cases of small bowel perforation, ischaemic gastritis and pancreatitis have also been reported.26
Stool microscopy and culture should be performed to exclude infectious causes of diarrhoea.28 Computed tomography (CT) scans can detect features of colitis,29 and lower gastrointestinal endoscopy with colonic biopsy may help confirm or exclude colitis.30 Severe colitis is uncommon but can be complicated by large-bowel obstruction or perforation. Therefore, urgent and thorough assessment of diarrhoea is required for patients receiving ICIs.
Suggested management of diarrhoea and colitis is described in Box 5. Infliximab (5 mg/kg IV) can be effective in steroid-refractory cases and additional doses may be given at 2 weeks and 6 weeks if symptoms persist or recur.28,30 Tacrolimus or mycophenolate mofetil have also been suggested for refractory disease.5,30 Surgical intervention might be required in selected cases.
Hepatotoxicity
Immune-mediated hepatitis causing abnormal liver function tests occurs quite frequently with ICIs, particularly with combination therapy (Box 2). Fulminant hepatitis causing liver failure is rare.30 Raised levels of alanine aminotransferase or aspartate aminotransferase, with or without raised bilirubin levels, may be detected in asymptomatic patients. Therefore, routine liver function testing should precede each ICI dose. Other causes of liver dysfunction should be considered, particularly viral hepatitis, hepatotoxic drugs, alcohol or liver metastases. The reported radiological features by magnetic resonance imaging, CT scan or ultrasonography are varied, but imaging can be helpful to exclude other pathology.31 Liver biopsy may also be necessary to exclude alternative diagnoses.30
Management of hepatitis is outlined in Box 5. Severe, steroid-refractory hepatitis may respond to additional immunosuppressive therapy (eg, mycophenolate mofetil 500–1000 mg twice daily).30 Successful use of anti-thymocyte globulin has been reported in at least two cases.32,33 Infliximab is not recommended owing to potential hepatotoxicity.6
Endocrinopathies
The most frequent endocrinopathies related to ICIs are thyroid dysfunction and hypophysitis (Box 2). Rare cases of primary adrenal insufficiency and type 1 diabetes mellitus have also been reported. In contrast to other irAEs, endocrinopathies are usually irreversible and require long term hormone replacement.
Transient, asymptomatic elevation of thyroid-stimulating hormone occurs in some patients and may progress to hypothyroidism. Hypothyroidism is more commonly associated with anti-PD-1 therapy compared with ipilimumab.7,9 Hyperthyroidism is less common and may be due to transient thyroiditis preceding hypothyroidism. Thyroid dysfunction management is summarised in Box 5.
While sporadic autoimmune hypophysitis is very rare, hypophysitis with hypopituitarism is an important endocrine complication of ICI use, particularly ipilimumab.34 Magnetic resonance imaging scans demonstrate typical changes of diffuse pituitary enlargement, and hormone assessment may reveal hypoadrenalism, hypothyroidism and hypogonadism.35 Asymptomatic hypophysitis requires hormone replacement as directed by an endocrinologist. However, if symptoms such as headache or visual disturbance are present, high dose steroid treatment is recommended (prednisolone 1–2 mg/kg/day or IV equivalent) tapered over 2–4 weeks once improving, with continuation of long term hormone replacement required in most cases.35
Primary or secondary adrenal insufficiency can present as an adrenal crisis.6 This is an emergency and requires immediate treatment with IV corticosteroids (eg, hydrocortisone or methylprednisolone), along with IV fluids and supportive measures.6,19 Short term high dose steroids are continued until stable, and then weaned down to a physiological replacement dose.
Pulmonary toxicity
Pneumonitis is generally not common with ICIs (Box 2). Interestingly, the incidence is higher with anti-PD-1 therapy for lung cancer (3–5%) than for melanoma (1–2%).5 Symptoms include dry cough and dyspnoea. CT imaging shows ground glass or nodular lung infiltrates, and pulmonary function tests can be useful.36 Exclusion of infection is necessary, which may require bronchoscopy with bronchoalveolar lavage. Pneumonitis management is described in Box 5.
Rheumatological toxicity
Arthralgia and myalgia are relatively frequent with all ICIs, but are predominantly mild or moderately severe (Box 2). Cases of polyarticular inflammatory arthritis, myositis and vasculitis have been reported, but these are rare.5,26 Mild arthralgia or myalgia can be managed with simple analgesia (paracetamol or non-steroidal anti-inflammatory drugs), while moderate symptoms may require medium dose steroids (prednisolone 10–20 mg/day).5 Severe symptoms need high dose steroids (prednisolone 1 mg/kg/day) with rheumatology consultation to consider additional immunosuppressive therapy.
Neurological toxicity
Neurological irAEs are uncommon (Box 2), although high grade events have been reported rarely, including myasthenia gravis, chronic inflammatory demyelinating polyneuropathy, transverse myelitis and Guillain-Barré syndrome.26,37,38 ICIs should be withheld and neurological consultation obtained in such cases. Corticosteroids have been used effectively for ICI-related myasthenia gravis and Guillain-Barré syndrome, although intravenous immunoglobulin or plasmapheresis may be necessary in steroid-refractory cases.5,37
Peripheral neuropathies have been reported, which may be sensory, motor or mixed.6,19 These are often transient and usually mild. Management of peripheral neuropathy is described in Box 5.
Cases of aseptic meningitis, cranial nerve palsy and posterior reversible encephalopathy syndrome have also been described.36
Nephrotoxicity
Acute kidney injury (AKI) with elevated serum creatinine level is uncommon with ICIs (Box 2). Cases of interstitial nephritis, granulomatous nephritis and lupus-like glomerulonephritis have been reported.36,39 ICIs can be continued with weekly creatinine monitoring for grade 1 AKI (creatinine levels 1.5 to 2 times baseline). For grade 2 AKI (creatinine 2 to 3 times baseline), ICI therapy should be withheld and steroids commenced (prednisolone 0.5–1 mg/kg/day). Grade 3 (creatinine levels > 3 times baseline) or grade 4 (life-threatening consequences or dialysis indicated) AKI requires high dose steroids (prednisolone 1–2 mg/kg/day or IV equivalent) and discontinuation of ICI therapy. Nephrology consultation and renal biopsy may be necessary to guide treatment.
Discussion
ICIs have improved the prognosis of patients with advanced cancer, with unprecedented survival rates seen in certain cancers such as advanced melanoma. However, their associated burden of toxicity is not insignificant. The incidence and spectrum of irAEs seen with expanded use appears to be similar to those recorded in the initial clinical trials.40–42 Some older studies suggested that the development of irAEs may be associated with better clinical response.43 However, this has not been a consistent finding, with immune-related toxicity and positive clinical response able to occur independently.3,5
Additional costs to the health care system are incurred from treatment-related toxicity and unplanned hospital admissions. Data on hospital admission rates related to irAEs are still limited; however, recent data from patients receiving combination anti-CTLA-4 and anti-PD-1 therapy showed that almost half required hospital admission for management of irAEs.44 Hospital admissions may be prolonged in some cases, although data are not available on the median duration of such admissions.
It may be possible to reduce these additional costs through early recognition and treatment of irAEs. Biomarkers to stratify irAE risk before treatment, or to predict irAEs before clinical disease emerges on treatment, would be valuable but are currently lacking. In the absence of such biomarkers, careful monitoring of clinical and laboratory signs of emerging irAEs is needed.
While algorithms similar to those outlined in this article are widely used, evidence from prospective studies would be valuable to optimise management. Further research is also required into the use of ICIs in patients with existing autoimmune disorders, as such patients have traditionally been excluded from clinical trials. Evidence from case reports and case series suggests that while some patients do tolerate ICIs, others experience flares of their underlying disease.5,45,46
The decision to resume ICI therapy or not following an irAE can be complicated, considering possible further serious toxicity with potential for sustained remission. Most patients have a poor prognosis if untreated and are often more willing to accept the risk of adverse events related to therapy.47 It is known that a high proportion of patients are unable to complete therapy due to irAEs, particularly with combination CTLA-4 and PD-1 inhibitor therapy.44 However, there is evidence that the overall survival of patients who discontinue ICIs because of irAEs is not reduced compared with patients who complete therapy.48
Use of anti-PD-1 therapy has been reported in patients with previous severe irAEs related to ipilimumab.46,49 Recurrence of the previous irAE appears to be rare in this situation; however, new irAEs may still occur.
A trial of planned sequential use of anti-CTLA-4 therapy followed by anti-PD-1 therapy, or the reverse, has also been reported.50 Nivolumab followed by ipilimumab showed better response but increased toxicity compared with the reverse sequence. In this trial, toxicity with one ICI did not predict risk of toxicity with a subsequent ICI. The frequency and kinetics of irAEs with sequential ICI therapy may be different to single-agent therapy.51
Early symptoms of toxicity can be non-specific; therefore, patients may initially present to general practitioners or to local hospitals that have less familiarity with ICIs and irAEs. Even within tertiary care hospitals, doctors practising outside of medical oncology often lack awareness of these drugs and their potential toxicities. However, owing to the diverse manifestations of irAEs, many different specialties will encounter patients with such problems. Strategies that prompt health care providers to consider the possibility of irAEs, such as medical alert cards or electronic alerts, should be part of routine management, along with education of patients and their carers to recognise signs of toxicity.21
Individual institutions should consider developing modified guidelines that incorporate local practices and expertise. Improving general awareness of ICI toxicities and establishing a standardised, multidisciplinary approach to the treatment of irAEs may reduce the associated morbidity and mortality. Anticipating and preparing for rare but potentially serious complications is important. For example, access to high cost drugs for off-label indications may be difficult in some settings; therefore, forward planning with the hospital’s drug and therapeutics committee can expedite access to such drugs for urgent treatment of irAEs.
The development of ICIs has certainly strengthened the arsenal available to medical oncologists in the fight against several advanced cancers. Optimising management of irAEs will limit the collateral damage and hopefully further improve disease outcomes and quality of life for patients.
Box 1 –
Mechanism of action of immune checkpoint inhibitors
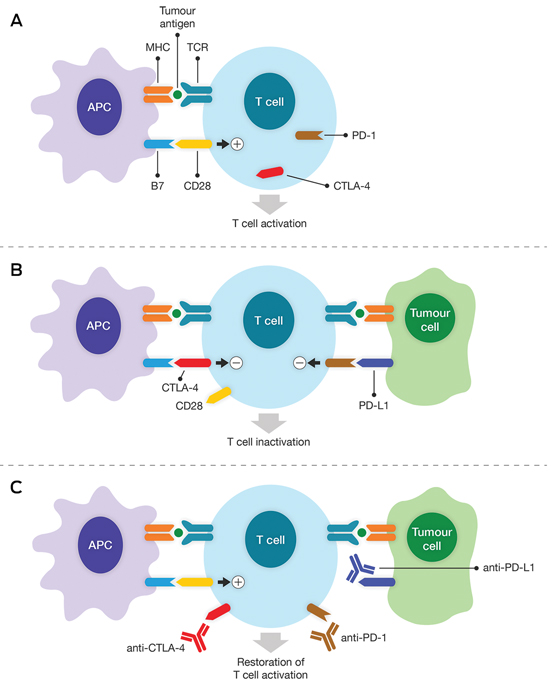
A. Activation of tumour-specific cytotoxic T cells first requires interaction with the appropriate antigen. The tumour antigen is displayed by the major histocompatibility complex (MHC) on the surface of antigen-presenting cells (APC), which can then bind to the T cell receptor (TCR). Full T cell activation also requires a co-stimulatory signal, which is provided by B7 molecules on the APC and binding to CD28 molecules on the T cell. B. Following T cell activation, inhibitory receptors such as cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) are expressed on the T cell surface to regulate the immune response. CTLA-4 binds to B7 molecules with higher affinity than CD28 molecules and transmits an inhibitory signal to the T cell, leading to inactivation. PD-1 binds to its ligand PD-L1, which may be expressed by tumour cells. An inhibitory signal is also transmitted via PD-1, which causes T cell inactivation and prevents the immune response against the tumour. C. These inhibitory signals can be blocked using therapeutic monoclonal antibodies (known as immune checkpoint inhibitors). Anti-CTLA-4 antibodies prevent B7–CTLA-4 binding, and allow the co-stimulatory signal via B7–CD28 binding to be restored. Anti-PD-1 and anti-PD-L1 antibodies disrupt the inhibitory signal to the T cell via PD-1. The result in all cases is restoration of T cell activation and the tumour-specific immune response.
Box 2 –
Incidence of immune-related adverse events associated with immune checkpoint inhibitors7–14
|
Immune-related adverse event |
|
|
|
|
|||||||||||
|
All grades |
Grade 3 or 4 |
All grades |
Grade 3 or 4 |
All grades |
Grade 3 or 4 |
All grades |
Grade 3 or 4 |
||||||||
|
|
|||||||||||||||
|
Dermatological |
|
|
|
|
|
|
|
|
|||||||
|
Rash |
15–33% |
0–2% |
28–41% |
3–5% |
4–26% |
0–1% |
10–15% |
< 1% |
|||||||
|
Pruritus |
25–35% |
0–2% |
33–35% |
1–2% |
6–19% |
0–1% |
11–14% |
0 |
|||||||
|
Vitiligo |
2–9% |
0 |
7–11% |
0 |
7–11% |
0 |
9–11% |
0 |
|||||||
|
Gastrointestinal |
|
|
|
|
|
|
|
|
|||||||
|
Diarrhoea |
23–37% |
3–11% |
44–45% |
9–11% |
8–19% |
0–3% |
8–17% |
1–3% |
|||||||
|
Colitis |
8–13% |
7–9% |
12–23% |
7–8% |
1% |
< 1% |
1–4% |
1–3% |
|||||||
|
Hepatitis |
1–4% |
0–2% |
22–30% |
11–19% |
1–6% |
0–3% |
1–3% |
0–2% |
|||||||
|
Endocrine |
|
|
|
|
|
|
|
|
|||||||
|
Hypothyroidism |
2–15% |
0 |
15–16% |
< 1% |
4–9% |
0 |
8–10% |
< 1% |
|||||||
|
Hyperthyroidism |
1–2% |
< 1% |
10% |
1% |
2–4% |
< 1% |
2–4% |
0 |
|||||||
|
Hypophysitis |
2–7% |
2–4% |
8–12% |
2% |
< 1% |
< 1% |
< 1% |
< 1% |
|||||||
|
Pneumonitis |
0–4% |
0–2% |
6–11% |
1–2% |
1–5% |
0–3% |
0–5% |
0–2% |
|||||||
|
Rheumatological |
|
|
|
|
|
|
|
|
|||||||
|
Myalgia |
2–13% |
< 1% |
10% |
0 |
2–6% |
0–1% |
2–7% |
< 1% |
|||||||
|
Arthralgia |
5–9% |
< 1% |
11% |
< 1% |
5–8% |
0 |
9–12% |
< 1% |
|||||||
|
Arthritis |
0 |
0 |
nr |
nr |
nr |
nr |
0–2% |
0 |
|||||||
|
Neurological |
|
|
|
|
|
|
|
|
|||||||
|
Headache |
2–8% |
< 1% |
3–10% |
0–1% |
4–7% |
0 |
2–3% |
0 |
|||||||
|
Paraesthesia |
1% |
< 1% |
nr |
nr |
2% |
0 |
< 1% |
0 |
|||||||
|
Renal |
0–3% |
< 1% |
3–6% |
1–2% |
1–2% |
< 1% |
< 1% |
0 |
|||||||
|
Haematological |
|
|
|
|
|
|
|
|
|||||||
|
Anaemia |
< 1% |
< 1% |
nr |
nr |
2–4% |
1% |
1–3% |
0–1% |
|||||||
|
|
|||||||||||||||
|
nr = not recorded. |
|||||||||||||||
Box 4 –
Common Terminology Criteria for Adverse Events version 4.0 (CTCAE)*23
|
Grade |
Definition |
||||||||||||||
|
|
|||||||||||||||
|
1 |
Mild; or |
||||||||||||||
|
2 |
Moderate; or |
||||||||||||||
|
3 |
Severe or medically significant but not immediately life threatening; or |
||||||||||||||
|
4 |
Life-threatening consequences; or |
||||||||||||||
|
5 |
Death related to adverse event |
||||||||||||||
|
|
|||||||||||||||
|
* CTCAE displays grades 1 through 5 with unique clinical descriptions of severity for each individual adverse event based on this general guideline. † Instrumental activities of daily living refer to preparing meals, shopping for groceries or clothes, using the telephone, managing money, etc. ‡ Self-care activities of daily living refer to bathing, dressing and undressing, feeding self, using the toilet, taking medications, and not bedridden. |
|||||||||||||||
Box 5 –
Management of the most common immune-related adverse events (irAEs)
|
irAE |
Grade 1 (G1, mild) |
Grade 2 (G2, moderate) |
Grade 3 (G3, severe) |
Grade 4 (G4, life threatening) |
|||||||||||
|
|
|||||||||||||||
|
Rash |
< 10% BSA:
|
10–30% BSA:
|
> 30% BSA:
|
Life-threatening consequences; urgent intervention indicated:
|
|||||||||||
|
Diarrhoea or colitis |
< 4 bowel actions/day over baseline:
|
4-6 bowel actions/day over baseline; abdominal pain, mucous or blood in stool:
|
≥ 7 bowel actions/day over baseline; severe abdominal pain, peritoneal signs:
|
Life-threatening consequences; urgent intervention indicated:
|
|||||||||||
|
Hepatitis |
AST/ALT up to 3 times ULN and/or total BILI up to 1.5 times ULN:
|
AST/ALT 3–5 times ULN and/or total BILI 1.5–3 times ULN:
|
AST/ALT 5–20 times ULN and/or total BILI 3–10 times ULN:
|
AST/ALT > 20 times ULN and/or total BILI > 10 times ULN:
|
|||||||||||
|
Thyroid dysfunction |
Asymptomatic, intervention not indicated:
|
Symptomatic; therapy indicated:
|
Severe symptoms; hospitalisation indicated:
|
Life-threatening consequences; urgent intervention indicated:
|
|||||||||||
|
Pneumonitis |
Asymptomatic; intervention not indicated:
|
Symptomatic; intervention indicated:
|
Severe symptoms; oxygen indicated:
|
Life-threatening respiratory compromise; urgent intervention indicated:
|
|||||||||||
|
Neurological |
Asymptomatic or mild symptoms:
|
Moderate symptoms:
|
Severe symptoms:
|
Life-threatening symptoms:
|
|||||||||||
|
|
|||||||||||||||
|
ALT = alanine aminotransferase. AST = aspartate aminotransferase. BILI = bilirubin. BSA = body surface area. C/I = contraindicated. CYC = cyclophosphamide. ICI = immune checkpoint inhibitor. ID = infectious diseases. IV = intravenous. IVIG = intravenous immunoglobulin. LFTs = liver function tests. MMF = mycophenolate mofetil. TFTs = thyroid function tests. ULN = upper limit of normal. |
|||||||||||||||
Nutting allergies out
Exposing babies to peanuts and eggs may head off a lifetime of unpleasant and potentially deadly allergies.
As researchers puzzle over the proliferation of food and other allergies in Western populations, a high-level analysis of results from 146 studies has found that parents could reduce the risk of allergic reactions in their child to eggs and peanuts later in life by introducing but foods at an early stage.
They found that children introduced to eggs at four to six months of age were less likely to develop an allergy, as were those exposed to peanuts between four and 11 months.
But this inuring effect did not necessarily apply to other foods and substances.
The researchers said there was low certainty that feeding fish to babies early on would result in “reduced allergic sensitisation and rhinitis”.
Similarly, “there was high-certainty evidence that timing of gluten introduction was not associated with celiac disease risk, and timing of allergenic food introduction was not associated with other outcomes”.
While the conclusions are based on the findings of a large number of studies, the researchers were cautious about drawing any definitive conclusions.
“Certainty of evidence was downgraded because of imprecision of effect estimates and indirectness of the populations and interventions studied,” they said. “Timing of egg or peanut introduction was not associated with risk of allergy to other foods.”
Adrian Rollins
Microgeographic factors and patterns of aeroallergen sensitisation
The known Socio-economic and geographic factors influence allergic sensitisation.
The new Coastal proximity, climate, and environmental (urban v regional) factors affect allergy patterns in the Greater Sydney area. A selected ten-aeroallergen skin prick test (SPT) panel identified 98.5% of atopic patients in our sample. 72.4% of grass-sensitised patients were co-sensitised to both temperate and subtropical grasses.
The implications The identified patterns of allergic sensitisation can inform more effective aeroallergen avoidance strategies. The high level of co-sensitisation to temperate and subtropical grasses suggests that existing immunotherapy is suboptimal in the Australian setting. Our selected Australian SPT panel may assist clinicians screening for allergy.
Allergic sensitisation is the first step in the pathogenesis of allergic disease. Socio-economic and geographic factors, including rural environment and climate, affect patterns of allergic sensitisation.1,2 Recognising sensitisation patterns may inform more effective allergen avoidance strategies, and help guide approaches to testing for and treating allergies. In particular, it may influence the choice of immunotherapy, a treatment modality that reduces symptoms and medication use, and which modifies disease in the long term.3
Our study explored airborne allergen (aeroallergen) sensitisation patterns in the Greater Sydney area, and their relationships with climate, proximity to the coast, and environment (urban or rural). As co-sensitisation patterns may be important when making choices about allergy testing and immunotherapy, we also explored patterns of co-sensitisation to temperate and tropical grasses, and to two house dust mite (HDM) species, Dermatophagoides farinae and Dermatophagoides pteronyssinus.
Methods
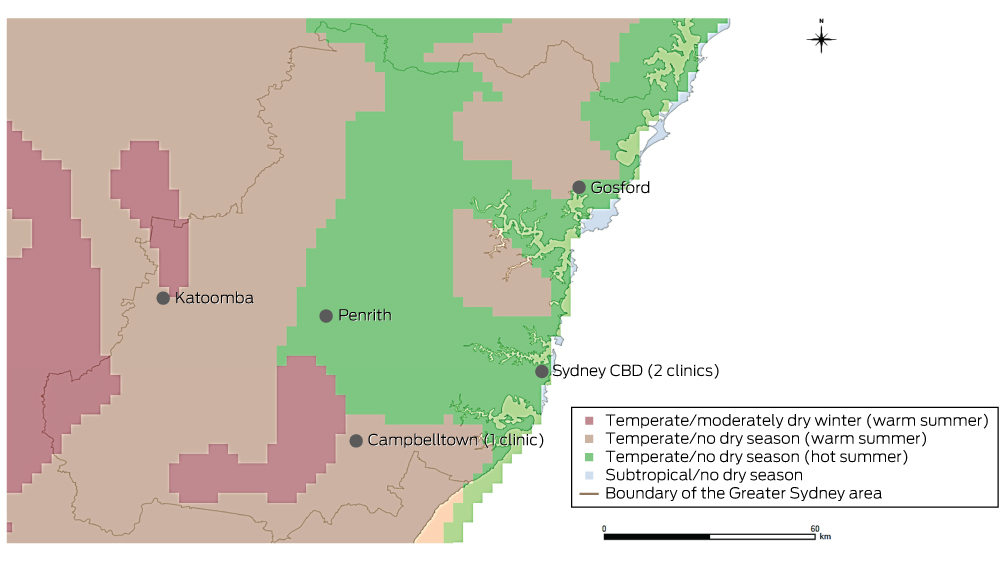
In a retrospective, cross-sectional, multicentre study, we analysed the electronic database records for patients of three Sydney allergy clinics who had undergone aeroallergen skin prick testing (SPT) during the period January 2001 – October 2014. Electronic records were available from January 2001 for one clinic, from January 2009 for the second, and from January 2013 for the third. One author (AWK) reviewed the records and extracted data on sex, date of birth, SPT results, and age and postcode at the time of testing. If patients had undergone multiple SPT, only the most recent results were analysed. Patients were excluded if their postcodes were outside the Greater Sydney area (hereafter: “Sydney”) as defined by the Australian Bureau of Statistics’ (ABS) Australian Statistical Geographic Standard4 (Box 1) or if their most recent SPT test was invalid.
Skin prick testing
Each clinic performed SPT according to the Australasian Society of Clinical Immunology and Allergy guidelines.5 SPT results were reported as the mean diameter (mm) of the wheal reaction to the testing reagent. Criteria for valid tests were that the mean diameter of the wheal induced by the negative (ie, non-allergenic) control, phenolated glycerol saline, was no greater than 3 mm, and that the wheal induced by the positive control, 10 mg/mL histamine dihydrochloride (directly elicits a cutaneous wheal and flare response), was more than 4 mm wider than that of the negative control.5 Patients were deemed sensitised to an allergen if the mean diameter of the induced wheal was at least 3 mm if there was no reaction to the negative control, or more than 3 mm larger than that of the negative control if there was a reaction to the negative control.5
The allergens tested were those known to be present in Sydney. We grouped these allergens according to shared characteristics: HDM (D. farinae, D. pteronyssinus); animals (cat, dog); cockroach mix; moulds (Aspergillus, Alternaria, Cladosporium, Penicillium); weeds (plantain, Parietaria, Oleaceae mix); trees (plane tree, pine tree mix [Pinus contorta and P. ponderosa], birch tree); temperate grasses (five grass mix [timothy, rye, meadow, sweet vernal, cocksfoot], rye grass, timothy grass); and tropical grasses (Bermuda grass, Bahia grass). HollisterStier Allergy and Stallergenes (Alyostal) reagents were used during testing. Some patients were exposed to different SPT panels, as clinically indicated at presentation.
Definition of coastal habitation
Each patient was assigned to one of three coastal habitation groups according to the distance of their postcode from the coastline (< 15 km, 15–30 km, > 30 km). To assign these groups, a map of ABS postcode areas6 (vector format) was opened in ArcGIS (ESRI), a geographic information system program. Each postcode area was converted to a point on the map determined by its geometric centroid (a single coordinate representing the average of all points in the postcode area). The distance of the centroid of each postcode from the nearest point on the coastline was then determined.
Climate zone classification
Climate zones were defined according to the Australian Köppen climate classification of the Australian Bureau of Meteorology (Appendix 1, Box 1).7 Four climate zones were defined in Sydney: subtropical/no dry season; temperate/no dry season (hot summer); temperate/no dry season (warm summer), and temperate/moderately dry winter (warm summer).7,8
To determine the climate zone of each postcode, a digitised map (vector format) of ABS postcode areas6 was overlaid with a digitised map (raster format) of the Australian Köppen climate zones in ArcGIS (ESRI).9 Postcodes were assigned the predominant climate zone (by area) within their boundaries.
Definition of urban and regional areas
Urban and regional areas were defined according to the Accessibility/Remoteness Index of Australia+ (ARIA+), an Australian government-endorsed geographic measure of remoteness.10 Two remoteness categories were defined in the Sydney area, “Major cities of Australia” (in this article: “urban”) and “Inner regional Australia” (“regional”).
Data analysis
The proportions of patients sensitised and co-sensitised to the tested aeroallergens were calculated. To determine the ten-aeroallergen panel that provided the highest detection rate of atopic individuals, descriptive analyses assessed all possible aeroallergen combinations. These analyses were performed in SPSS 22.0 (IBM). Confidence intervals for proportions of patients exhibiting aeroallergen sensitisation were calculated by the Clopper and Pearson method in GraphPad Prism 6.04 (GraphPad Software).
Differences in the proportions of positive SPT results between climate zones, coastal and inland areas, and urban and regional areas were analysed in χ2 tests. Z-tests (with Bonferroni correction) identified significant pairwise differences between specific climate zones or coastal habitation areas. Analysis of data for coastal habitation areas by χ2 tests was adjusted for climate zone to assess the interaction of their effects. Sub-analysis of the 563 patients aged 16 years or less evaluated the influence of changes of address on results; it was assumed that children were less likely to have moved house as often as adults. These analyses were performed in SPSS 22.0.
Ethics approval
Ethics approval was granted by the St Vincent’s Hospital Human Research Ethics Committee (reference, LNR/14/SVH/88).
Results
A total of 1421 patients met the selection criteria. The mean age at testing was 28.3 years (SD, 21.3); 757 patients were female (53.3%). As expected of tertiary allergy services, there was a high proportion of sensitised patients, with 1092 (76.8%; 95% confidence interval [CI], 74.6–79.0%) sensitised to at least one aeroallergen. The distribution of patients between climatic and geographic zones is summarised in Appendix 2.
Across Sydney, the most common sensitising aeroallergens were HDM (63.2% of tested patients; 95% CI, 60.6–65.7%) and grasses (46.3%; 95% CI, 43.6–49.0%). Sensitisation to temperate grasses (44.5%; 95% CI: 41.7–47.4%) was more common than to subtropical grasses (37.6%; 95% CI, 34.7–40.4%; P < 0.001). The most common sensitising aeroallergens are listed in Box 2.
Among the 1092 patients sensitised to at least one aeroallergen, 1057 (96.8%) had undergone both HDM and grass pollen SPT; of these, 995 (94.1%) were sensitised to at least one of these allergen groups, and 484 (45.8%) were sensitised to both HDM and grass pollen.
Of the 901 patients who had undergone testing to all eight allergen groups, 124 (13.8%) were sensitised to only one allergen group (mono-sensitised), of whom 72% were mono-sensitised to HDM aeroallergen (Box 3).
Climate zone influence
There were no climate zone differences in the patterns of HDM and animal aeroallergen sensitisation. A temperate/warm summer climate, however, was associated with a higher proportion of patients sensitised to cockroach, mould, weed, tree, and temperate or subtropical grass allergens than were temperate/hot summer and subtropical climates (Box 4).
A sub-analysis of patients aged 16 years or younger found that HDM sensitisation was more common in temperate/hot summer climates (72.9%) than in temperate/warm summer climates (moderately dry winter, 51.8%; no dry season, 59.6%; P < 0.001). There were no significant differences in patterns of sensitisation between climate zones for other allergen groups (data not shown).
Coastal habitation
There was no relationship between coastal proximity and patterns of HDM and cat sensitisation. Lower proportions of patients residing less than 15 km from the coast were sensitised to cockroach, mould, weed, tree, temperate grass and subtropical grass aeroallergens than those further inland (Box 5, Appendix 3).
All participants living in temperate/moderately dry winter, warm summer zones also lived more than 30 km from the coast, while all in subtropical climate zones lived more than 15 km from the coast. In both temperate/no dry season zones, the pattern of increasing sensitisation further from the coastline remained significant after adjustment for climate zone, with two exceptions: there was no relationship between coastal proximity and sensitisation to cockroach and mould in patients living in temperate/no dry season, warm summer climates (data not shown).
A sub-analysis of tested patients aged 16 years or younger indicated that those residing less than 15 km from the coast were less commonly sensitised to mould, weed, tree and subtropical grass aeroallergens than patients further inland (P < 0.05 for each). Similar patterns were observed for cockroach and temperate grass sensitisation, but were not statistically significant (P = 0.063 and P = 0.055 respectively) (data not shown).
Urban and regional residence
Lower proportions of patients in urban areas were sensitised to cockroach, mould and subtropical grass aeroallergens than in regional areas. There were no differences for other allergen groups (Box 6). Although the differences in a sub-analysis of patients aged 16 years or younger were similar, they were not statistically significant (cockroach, P = 0.153; moulds, P = 0.456; subtropical grasses, P = 0.190) (data not shown).
Co-sensitisation
A total of 1135 patients underwent both temperate and subtropical grass SPT. Of the 554 sensitised to any grass, 401 (72.4%) were sensitised to both temperate and subtropical grasses; 128 patients (23.1%) were sensitised to temperate grasses only, and 25 (4.5%) to subtropical grasses only.
A total of 496 patients underwent both D. pteronyssinus and D. farinae SPT. Of the 301 sensitised to HDM, 260 (86.4%) were sensitised to both species, while 32 patients (11%) were sensitised to D. pteronyssinus only and 9 (3%) to D. farinae only.
Testing panel
A ten-aeroallergen testing panel consisting of D. pteronyssinus, cat, dog, cockroach mix, Alternaria, Aspergillus, plantain, rye grass, timothy grass, and either Bermuda or Bahia grass (the two grasses yielded the same results) identified 98.5% of all sensitised patients (1076 of 1092).
Discussion
This study explored patterns of aeroallergen sensitisation in Sydney. Other authors have hypothesised that D. pteronyssinus is the predominant HDM allergen in Sydney;11 indeed, it has been found that D. farinae comprises only 5.1% of HDMs found in house dust in Sydney.12 Other studies of HDM species prevalence reported that D. farinae is rarely found in Australian cities, suggesting the predominance of D. pteronyssinus across the nation,13–15 an interpretation supported by our results. Only a small proportion of our Sydney sample were sensitised solely to D. farinae (3%) and a comparatively higher proportion to D. pteronyssinus alone (11%). D. farinae and D. pteronyssinus allergen cross-reactivity probably explains the high proportion of HDM-sensitised patients who were co-sensitised to both species (86.4%).
A panel of ten aeroallergens — D. pteronyssinus, cat, dog, cockroach mix, Alternaria, Aspergillus, plantain, rye grass, timothy grass, and either Bermuda or Bahia grass — may be a useful screening panel in Sydney, having identified 98.5% of atopic patients in our study. This panel, modified by adding or subtracting aeroallergens according to regional variations in the predominance of allergens, may also be useful elsewhere in Australia.
We found a high proportion of co-sensitisation to temperate and subtropical grasses in the Sydney area (72.4%), consistent with findings by studies in subtropical Australian regions.16 This differs from findings in Europe and North America, where temperate grasses are predominantly responsible for grass pollen sensitisation.17,18 Temperate and subtropical grass pollens have both shared and distinct immunological properties, and the IgE reactivity of residents of temperate and subtropical regions is higher to the grass group of the corresponding climate.16,19,20 This suggests that differences in temperate and subtropical grass immunological reactivity are clinically relevant. As such, the high proportion of patients co-sensitised to temperate and subtropical grasses which we found indicates that both temperate and subtropical grass testing and immunotherapy may be important in Sydney and other temperate and subtropical regions. As most immunotherapeutic agents, particularly newer sublingual immunotherapy tablets, are directed against temperate grass aeroallergens alone, they may not provide adequate coverage in Australia.16,21 Treatments directed against both temperate and subtropical grasses may be required. Bermuda grass testing and immunotherapy may be particularly relevant, as it is less cross-reactive with temperate grasses than Bahia grass.20
Our results confirmed that sensitisation to Alternaria, grass, weed and tree is less common in residents near the coast.11,22,23 Further, cockroach, Aspergillus and Cladosporium sensitisation is less common in areas less than 15 km from the sea. The climatic conditions of the coast (including increased humidity, thermal stability, and coastal breezes) may offer some protection against these aeroallergens.
Interestingly, regional patients were more frequently sensitised to cockroach than were urban residents. A possible explanation is that our “regional” areas lay on the outskirts of Sydney, and were areas with lower household incomes and socio-economic status,24 both of which are risk factors for sensitisation to cockroach aeroallergens.25,26
The strengths of our study included the fact that it was the first to examine associations between sensitisation to specific allergen groups and climatic and geographic factors in an Australian setting over distances of tens of kilometres. It is also the first to report the prevalence of aeroallergen sensitisation in the Sydney area in a cohort of this size. Further, by separately analysing sensitisation for those under 16 years of age, who were unlikely to have changed addresses as often as older people, the study provides strong evidence of the effect of coastal habitation on reducing sensitisation rates to mould, weed, tree and subtropical grass aeroallergens. Finally, this study is more likely to reflect patterns of clinically relevant sensitisation because it used SPT results to define sensitisation, rather than allergen-specific IgE blood tests; further, it was restricted to patients who had presented to allergy specialists.
Our study, however, has limitations. Firstly, as postcodes and not exact addresses defined places of residence, microclimate and geographic location could not be precisely allocated for each person. Secondly, the proportion of people with allergic sensitisation in our investigation would be higher than in the general population, as we studied patients attending tertiary allergy clinics. Finally, as some patients were tested with different SPT panels, according to clinical indication at presentation, the prevalence of sensitisation we report may be higher than if all patients had undergone SPT for each of the allergens. This limitation was unavoidable, given the retrospective nature of our study. We also note that clinical reactivity requires both allergen sensitisation and subsequent allergen exposure. As such, sensitisation is clinically relevant only when there is also a risk of exposure to the allergen.
In conclusion, our study yields three major insights. Firstly, allergic sensitisation to a variety of different aeroallergens was less common in patients attending allergy clinics who resided less than 15 km from the coast, in temperate/hot summer or subtropical climates, or in urban parts of Sydney. These relationships may affect decisions about allergen reduction and avoidance measures. Secondly, a ten-aeroallergen SPT panel identified more than 98% of atopic patients in Sydney; this panel may assist clinicians when screening for allergy. Finally, currently available immunotherapeutic options, based on northern hemisphere temperate grass allergens, may be inappropriate in the Australian setting in view of the high degree of co-sensitisation to temperate and subtropical grasses; regimens directed at both grass types would be more suitable for treating grass allergy in subtropical regions.
Box 2 –
Proportion of patients exhibiting sensitisation to specific aeroallergens
|
Allergen |
Number of patients tested |
Number sensitised |
Proportion sensitised (95% CI) |
||||||||||||
|
|
|||||||||||||||
|
House dust mite |
1404 |
887 |
63.2% (60.6–65.7) |
||||||||||||
|
D. farinae |
496 |
269 |
54.2% (49.7–58.7) |
||||||||||||
|
D. pteronyssinus |
1404 |
878 |
62.5% (59.9–65.1) |
||||||||||||
|
Animals |
1303 |
517 |
39.6% (37.2–42.1) |
||||||||||||
|
Cat |
1274 |
452 |
35.5% (32.8–38.2) |
||||||||||||
|
Dog |
1132 |
256 |
22.4% (20.2–24.7) |
||||||||||||
|
Cockroach mix |
983 |
295 |
30.0% (27.2–33.0) |
||||||||||||
|
Moulds |
1295 |
347 |
26.8% (24.4–29.3) |
||||||||||||
|
Alternaria |
1242 |
255 |
20.5% (18.3–22.9) |
||||||||||||
|
Aspergillus |
1213 |
154 |
12.7% (10.9–14.7) |
||||||||||||
|
Cladosporium |
872 |
137 |
15.7% (13.4–18.3) |
||||||||||||
|
Penicillium |
628 |
76 |
12.1% (9.7–15) |
||||||||||||
|
Weeds |
1207 |
387 |
32.1% (29.4–34.8) |
||||||||||||
|
Plantain |
1204 |
340 |
28.2% (25.7–30.9) |
||||||||||||
|
Parietaria |
636 |
27 |
4.2% (2.8–6.1) |
||||||||||||
|
Oleaceae mix |
634 |
68 |
10.7% (8.4–13) |
||||||||||||
|
Trees |
972 |
155 |
15.9% (13.7–18.4) |
||||||||||||
|
Plane tree |
932 |
65 |
7.0% (5.4–8.8) |
||||||||||||
|
Pine mix |
564 |
33 |
5.9% (4.1–8.1) |
||||||||||||
|
Birch tree |
616 |
116 |
18.8% (15.8–22.1) |
||||||||||||
|
Temperate grass |
1209 |
538 |
44.5% (41.7–47.4) |
||||||||||||
|
Five grass mix |
188 |
68 |
36.2% (29.3–43.5) |
||||||||||||
|
Rye grass |
1103 |
490 |
44.4% (41.5–47.4) |
||||||||||||
|
Timothy grass |
605 |
328 |
54.2% (50.1–58.2) |
||||||||||||
|
Subtropical grass |
1137 |
427 |
37.6% (34.7–40.4) |
||||||||||||
|
Bermuda grass |
1129 |
383 |
33.9% (31.2–36.8) |
||||||||||||
|
Bahia grass |
755 |
331 |
43.8% (40.3–47.5) |
||||||||||||
|
All grasses |
1350 |
625 |
46.3% (43.6–49.0) |
||||||||||||
|
Any aeroallergen |
1421 |
1092 |
76.8% (74.6–79.0) |
||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 3 –
Aeroallergen groups to which 124 mono-sensitised patients were sensitised*
|
Allergen group |
Number mono-sensitised (%) |
||||||||||||||
|
|
|||||||||||||||
|
House dust mite |
89 (72%) |
||||||||||||||
|
Animals |
8 (6%) |
||||||||||||||
|
Cockroach |
3 (2%) |
||||||||||||||
|
Moulds |
11 (9%) |
||||||||||||||
|
Weeds |
4 (3%) |
||||||||||||||
|
Trees |
1 (1%) |
||||||||||||||
|
Temperate grasses |
8 (6%) |
||||||||||||||
|
Subtropical grasses |
0 |
||||||||||||||
|
|
|||||||||||||||
|
* This table includes only patients who had undergone skin prick testing for all eight allergen groups (901 patients) and who were sensitised to aeroallergens of only one allergen group. |
|||||||||||||||
Box 4 –
Aeroallergen sensitisation according to climate zone of residence
|
Allergen |
Temperate/moderately dry winter, warm summer |
Temperate/no dry season, warm summer |
Temperate/no dry season, hot summer |
Subtropical |
P† |
||||||||||
|
Sensitised (%) |
Number tested |
Group* |
Sensitised (%) |
Number tested |
Group* |
Sensitised (%) |
Number tested |
Group* |
Sensitised (%) |
Number tested |
Group* |
||||
|
|
|||||||||||||||
|
House dust mite |
58% |
116 |
— |
63.2% |
476 |
— |
64.4% |
781 |
— |
52% |
31 |
— |
0.29 |
||
|
Animals |
33% |
106 |
— |
42.1% |
416 |
— |
39.6% |
750 |
— |
32% |
31 |
— |
0.30 |
||
|
Cockroach |
38% |
84 |
α |
38.5% |
361 |
α |
24.1% |
514 |
β |
0 |
24 |
γ |
< 0.001 |
||
|
Mould |
29% |
102 |
α, β |
36.3% |
419 |
β |
22.0% |
744 |
α, γ |
3% |
30 |
γ |
< 0.001 |
||
|
Weeds |
37% |
83 |
α, β |
42.6% |
371 |
β |
26.6% |
723 |
α |
20% |
30 |
α, β |
< 0.001 |
||
|
Trees |
13% |
85 |
α, β |
24.6% |
362 |
β |
11.0% |
501 |
α |
0 |
24 |
α |
< 0.001 |
||
|
Temperate grasses |
46% |
109 |
α, β |
53.6% |
435 |
β |
39.3% |
638 |
α, γ |
15% |
27 |
γ |
< 0.001 |
||
|
Subtropical grasses |
50% |
93 |
α |
52.3% |
398 |
α |
27.1% |
619 |
β |
15% |
27 |
β |
< 0.001 |
||
|
All grasses |
50% |
111 |
α, β |
55.2% |
440 |
β |
41.9% |
768 |
α |
16% |
31 |
γ |
< 0.001 |
||
|
Total patients tested |
119 |
|
|
483 |
|
|
788 |
|
|
31 |
|
|
|||
|
|
|||||||||||||||
|
* Z-test (with Bonferroni corrections) for pairwise comparison of proportions of patients sensitised to each allergen group in each climate zone. Significant differences (P < 0.05) are indicated by allocating climate zones different Greek letters: α zones are significantly different from β and γ zones, but not from other α zones. For example, the proportion of patients sensitised to grass allergens in temperate/moderately dry winter, warm summer zones (50%) was significantly different to the proportion for subtropical zones (16%), but not the proportions in the other two temperate zone categories (55.2%, 41.9%). † χ2 test. |
|||||||||||||||
Box 5 –
Aeroallergen sensitisation in according to coastal proximity of residence
|
Allergen |
< 15 km from coastline |
15–30 km from coastline |
> 30 km from coastline |
P† |
|||||||||||
|
Sensitised (%) |
Number tested |
Group* |
Sensitised (%) |
Number tested |
Group* |
Sensitised (%) |
Number tested |
Group* |
|||||||
|
|
|||||||||||||||
|
House dust mite |
62.6% |
621 |
— |
66.0% |
468 |
— |
60.0% |
315 |
— |
0.22 |
|||||
|
Animals |
40.5% |
612 |
— |
36.8% |
410 |
— |
42.0% |
281 |
— |
0.33 |
|||||
|
Cockroach |
15.1% |
391 |
α |
40.0% |
355 |
β |
39.7% |
237 |
β |
< 0.001 |
|||||
|
Mould |
17.4% |
610 |
α |
37.2% |
406 |
β |
32.3% |
279 |
β |
< 0.001 |
|||||
|
Weeds |
23.2% |
611 |
α |
36.9% |
355 |
β |
47.3% |
241 |
γ |
< 0.001 |
|||||
|
Trees |
24.8% |
613 |
α |
39.2% |
380 |
β |
50.0% |
256 |
γ |
< 0.001 |
|||||
|
Temperate grass |
33.9% |
496 |
α |
49.9% |
421 |
β |
54.8% |
292 |
β |
< 0.001 |
|||||
|
Subtropical grass |
20.4% |
491 |
α |
46.1% |
384 |
β |
57.3% |
262 |
γ |
< 0.001 |
|||||
|
All grasses |
36.5% |
619 |
α |
52.2% |
435 |
β |
58.1% |
296 |
β |
< 0.001 |
|||||
|
Total number of patients tested |
676 |
|
|
472 |
|
|
323 |
|
|
||||||
|
|
|||||||||||||||
|
* Z-test (with Bonferroni corrections) for pairwise comparison of proportions of patients sensitised to each allergen group in each climate zone. Significant differences (P < 0.05) are indicated by allocating climate zones different Greek letters: α zones are significantly different from β and γ zones, but not from other α zones. † χ2 test. |
|||||||||||||||
Box 6 –
Aeroallergen sensitisation in urban and regional areas
|
Allergen |
Urban |
Regional |
P* |
||||||||||||
|
Sensitised (%) |
Number tested |
Sensitised (%) |
Number tested |
||||||||||||
|
|
|||||||||||||||
|
House dust mite |
63.1% |
1341 |
65% |
63 |
0.79 |
||||||||||
|
Animals |
39.5% |
1242 |
43% |
61 |
0.69 |
||||||||||
|
Cockroach |
29.1% |
932 |
47% |
51 |
0.011 |
||||||||||
|
Mould |
26.3% |
1238 |
39% |
57 |
0.047 |
||||||||||
|
Weeds |
31.6% |
1154 |
42% |
53 |
0.14 |
||||||||||
|
Trees |
15.8% |
923 |
18% |
49 |
0.69 |
||||||||||
|
Temperate grass |
44.0% |
1150 |
54% |
59 |
0.14 |
||||||||||
|
Subtropical grass |
36.7% |
1083 |
54% |
54 |
0.014 |
||||||||||
|
Grasses |
45.8% |
1289 |
56% |
61 |
0.15 |
||||||||||
|
Total number of patients tested |
|
1358 |
|
63 |
|
||||||||||
|
|
|||||||||||||||
|
* χ2 test. |
|||||||||||||||
Biosimilars in inflammatory bowel disease
Cost savings are welcome but evidence supporting equivalence of biosimilar and originator drugs is currently limited
The management of inflammatory bowel disease has undergone major changes in the last decade with the availability on the Pharmaceutical Benefits Scheme (PBS) of targeted biological therapies. The first of these was the anti-tumour necrosis factor α (anti-TNF-α) monoclonal antibody infliximab, followed by another anti-TNF-α antibody adalimumab, and, more recently, the first gut-specific T-cell trafficking inhibitor vedolizumab, an anti-α-4 β-7 integrin monoclonal antibody. These drugs have resulted in a shift in the management paradigm from symptom control and the minimisation of exposure to corticosteroids to now aiming for healing of the intestinal mucosa, prevention of damage and subsequent disability.1
The development of biologic medication is comparatively long and the manufacturing process very expensive, resulting in a high cost for these agents.2 In Australia, the most expensive single drug in absolute dollar value for the 2015 financial year was adalimumab, with biologic agents making up five of the top eight most costly drugs and accounting for over 12% of the total PBS spend.3 Given the increasing incidence of diseases that may be best managed by biologic agents, and the prolonged duration of therapy involved, the costs of these drugs are rising annually. These cost increases could pose a significant risk to the sustainability of the PBS system.
The patents for the initial biologic agents are starting to expire, which has led to the development of what are known as biosimilar versions of the originator product. These competitor drugs have created pressure to reduce the cost for the health system. The first biosimilar to infliximab was listed on the PBS in December 2015.
Biologic therapies are very different from chemically synthesised drugs, typically being large protein-containing agents produced from recombinant DNA and cell culture techniques, with complex post-translational modification and glycosylation. The technique of production can result in significant variability even between batches of production and requires strict quality assurance and in vitro assessments.4 The Therapeutic Goods Administration (TGA) has harmonised with and adopted a number of the guidelines of the European Medicines Agency regarding the assessment and approval of biosimilar medicines. To be considered a biosimilar medicine in Australia, the new product must have “demonstrable similarity in physicochemical, biological and immunological characteristics, efficacy and safety”.4 Despite this, these drugs are not considered to be identical or to have demonstrated bioequivalence with the originator biological medicine.
The major difference in the approval process between an originator drug and a biosimilar is that if the originator drug has more than one indication, the efficacy and safety of the biosimilar may only need to be demonstrated in one indication and this will be extrapolated to the other disease indications in which the originator drug is approved. The randomised controlled trials for the biosimilar infliximab CT-P13 (now commercially available in Australia) were only required in ankylosing spondylitis and rheumatoid arthritis and not Crohn disease or ulcerative colitis.5,6 The listing for the biosimilar infliximab on the PBS covers all the indications of the originator infliximab.
Cost savings accompany the listing of biosimilar agents on the PBS, with a mandated 16% drop in the PBS rebate and a move from the F1 to F2 formulary, where the drugs are then subject to application of the PBS price disclosure policy. This assesses the actual cost of supplying the drug to retail pharmacies and results in further adjustment and reduction of the PBS rebate over time to more accurately reflect the cost price.7
Despite the welcome cost reductions that have accompanied the arrival of the first biosimilars, unanswered questions remain about their long term interchangeability with the originator product. All biologics are immunogenic and can result in antibody formation, reactions, and loss of efficacy with time. Up to 20% of patients on maintenance therapy may develop antibodies to anti-TNF-α monoclonal antibodies.8 Given that biosimilar drugs are not identical, there is a theoretical risk that switching between agents may result in the development of neutralising anti-drug antibodies (ADAs) and subsequent loss of response. At present, there are some reassuring data from the open label extension studies of the PLANETAS and PLANETRA studies, where there were no significant differences in the rate of ADA formation between patients who continued on the biosimilar product and those who had a single switch from originator infliximab to the biosimilar infliximab at 1 year; however, trough drug levels were not reported.9,10 ADAs in the presence of low or absent drug levels are strongly associated with clinical loss of efficacy.11 Since the introduction of the biosimilar infliximab in Europe, several countries have mandated a single switch. The early data are reassuring but, as yet, only reported in small numbers and in abstract form, and a large Norwegian randomised controlled trial has recently been completed (https://clinicaltrials.gov/ct2/show/NCT02148640). Further reassuring data were seen in the cross-reactivity of ADA from patient sera to the originator infliximab having near identical binding to the biosimilar infliximab CT-P13; however, this has not been demonstrated in reverse.12 The vast majority of ADAs to anti-TNF-α monoclonal antibodies appear to be against the fragment antigen-binding region and may be neutralised by the addition of TNF-α. This reaction to TNF-α would be expected to be identical for originator and biosimilar agents and less prone to interference due to glycosylation and conformational changes.13
At present, studies have investigated a single switch between the originator and the biosimilar anti-TNF-α agent, whereas multiple switch and switch-back strategies have not been assessed. The Australian government has stated that biosimilar and originator anti-TNF-α agents can be considered interchangeable at the point of dispensing from the pharmacy, as is the case with small-molecule generic medicines. This practice known as “a-flagging” and requires patient consent.4 This may result in patients electing to receive a different anti-TNF-α agent at each time point of dispensing. Under the legislation, the only way a prescriber can ensure that a patient is continued on the initially prescribed biologic agent, be that either a biosimilar or an originator biologic, is to tick the “brand substitution not permitted” box on the prescription.
This decision has caused the greatest concern for prescribing clinicians and representative bodies. The absence of data to suggest adverse reactions or the development of ADA from multiple switching does not imply safety.
At the point of registration, biosimilars are required to satisfy the criteria of similarity to the originator, but the TGA has stated that it is “inevitable that reference and biosimilar medicines will diverge to some degree after comparability has been established”.14 In theory, this increases the chance of antigenic changes developing, and no clinical studies have assessed this to date. There has been no additional pharmacovigilance program instituted to monitor the outcomes of a-flagging, with only the drug sponsor required to develop a risk management plan and a reliance on voluntary reporting by prescribers of adverse outcomes to the TGA. This could be considered equivalent to conducting a clinical trial on the Australian public without the means to accurately capture data such as loss of response, requirement for corticosteroid therapy, or milder adverse reactions that may not result in reporting. However, a consultation process by the government on this with relevant stakeholders is ongoing.
Infliximab is only given intravenously, but the next biologic agents to come off patent that will result in biosimilars entering the market will be self-injectable (for example, etanercept and adalimumab for rheumatoid arthritis). The parenteral administration of these agents is far more complex than the taking of an oral agent and requires familiarity and ability to use the delivery devices. Further, many patients utilise and are dependent on patient support programs that are supplied by third party providers while being funded by the pharmaceutical companies. The viability of these important programs for patients who are potentially undergoing switches between biologic products is unknown.
A significant number of inflammatory bowel disease patients also require dose escalation to maintain clinical response, beyond the fixed dosing regimens funded by the PBS. At present, the compassionate access programs of the pharmaceutical companies provide these additional doses, with the PBS recently rejecting a submission to provide dose tailoring. With the potential for a patient to receive multiple versions of the same biologic, where there are no safety data supporting this practice, the provision of compassionate doses is also under threat.
The arrival of biosimilars is welcomed by clinicians for the cost savings they bring to our health system, but ongoing studies and pharmacovigilance are required in a framework that captures clinical response data. The decision to allow a-flagging in this area of limited evidence has caused concerns for clinicians and patients alike. Ongoing education of prescribers, pharmacists and patients is required, and the minimisation of unnecessary switches until more safety data are available is recommended.
Airline policies for passengers with nut allergies flying from Melbourne Airport
Australian flights carry 90 million passengers each year.1 About 1–2% of passengers have documented food allergies, of whom 2–10% report having experienced allergic reactions during air travel.2,3
Peanut and tree nut allergies are among the most serious of food allergies, and typically persist for life.4 The cornerstone of managing a food allergy is strict avoidance of the allergen. Should anaphylaxis occur, an intramuscular adrenaline injection may be life-saving.
During June and July 2015, we conducted a telephone and website survey of all domestic and international airlines that fly from Melbourne Tullamarine Airport to assess public access to airline nut allergy policies, the availability of nut-free meals and the ability to restrict the distribution of packaged nuts, and the in-flight availability of emergency adrenaline.
Of 33 airlines, 20 (61%) had accessible telephone information about a nut allergy policy, and this information was published on the websites of 20 airlines (61%). Telephone and website advice was discordant for three airlines, in that the customer service representatives advised that all nut allergies could be accommodated, but the website indicated that this applied only to peanuts.
Nine airlines (27%) offered nut-free meals, two routinely and seven on request (Box). For the other airlines, nut-allergic passengers would need to fast (only practical on short domestic routes) or bring their own food.
Twelve airlines (36%) could restrict the distribution of packaged nuts if requested, either totally or within an exclusion zone comprising the affected passenger’s row and the rows immediately in front of and behind them (Box). Four airlines (12%) could both offer a nut-free meal and restrict the distribution of packaged nuts.
People consuming packaged nuts place nut-allergic neighbours at risk of physical contact with nuts and accidental ingestion. Requesting nearby passengers not to consume nuts and wiping tray tables may reduce this risk.2 It is unclear whether nut-allergic individuals are at risk from airborne nut proteins, but instances of allergic reaction following such exposure have been reported.5
Only one airline confirmed that emergency adrenaline was available on all flights.
The diverse approaches by airlines to nut allergies reflect the lack of clear evidence supporting any specific policy. Airlines could nevertheless improve the access to relevant information. Given the discordant advice provided in some instances, the critical distinction between peanuts and tree nuts should be emphasised during customer service training. Carrying emergency adrenaline on all flights would seem prudent in light of the reported frequency of food allergy reactions.
We contacted only airlines flying from a single Australian airport, so that our survey is a representative sample of the industry rather than an exhaustive investigation. Telephone information from each airline was based on a single call, and the advice we received was current at the time of our telephone enquiries or website visit; in the meantime, airlines may have updated their policies.
We recommend that nut-allergic individuals contact airlines before travelling, develop an allergy management plan with their doctors, carry their own emergency medical supplies, and consider bringing their own food. Airlines should make their nut allergy policies more accessible and consider carrying emergency adrenaline on all flights.
Warnings for GPs over usage of flu vaccine in children
The Royal Australian College of General Practitioners has warned its members to be wary when administering the influenza vaccine to children under 5.
In a member communication, they have confirmed that there have been off-label usage of influenza vaccines Fluarix Tetra and Fluvax which poses a safety risk for children.
The RACGP says the GSK Fluarix Tetra is being incorrectly administered to children under 3 years (including half doses of the vaccine). The vaccine isn’t registered for this age group.
The Seqirus’ (BioCSL) Fluvax is also being incorrectly administered to children.
Related: Flu vaccine more effective in the morning: study
According to the Influenza Fact sheet from the Department of Health:
FluQuadri Junior® (Sanofi Pasteur) is for children from six months to under three years of age is presented in a 0.25 mL pre-filled syringe.
Fluarix® Tetra (GSK) for people aged three years and older is presented in a 0.5 mL pre-filled syringe.
In order to minimise vaccination accidents, the RACGP recommends:
- Ensure children’s vaccines are quarantined and clearly labelled in the vaccine fridge
- Ensure two clinical staff check each vaccine before administration
- Read the Australian Technical Advisory Group’s statement on influenza vaccines
- Discuss with your practice about practical steps to ensure patients receive the correct vaccine every time.
Latest news:
A patient with sarcoidosis and a cryptococcal infection of the skull
A 29-year-old man of European ancestry, with a history of sarcoidosis treated with low-dose steroids, presented with a progressive swelling of the skull which had appeared 3 weeks earlier.
Physical examination revealed a 2.5 cm soft swelling of the right parietal part of the skull. A contrast-enhanced magnetic resonance imaging scan of the head showed a solitary lesion of the right parietal bone measuring 9 mm × 17 mm.
After neurosurgical resection, pus cultures grew Cryptococcus neoformans.
In the absence of disseminated cryptococcosis, the patient was treated successfully with oral fluconazole. Current literature suggests sarcoidosis as a risk factor for cryptococcosis, independent of the use of immunosuppressive agents.1,2

 more_vert
more_vert