Cardiovascular disease (CVD) is the leading cause of morbidity and mortality internationally.1,2 A large proportion of CVD events are preventable by appropriate population-level interventions and individual management of risk. The potential for benefit, the balance of benefits and harms, and the cost-effectiveness of treatments to reduce CVD events are more closely related to an individual’s absolute CVD risk — the absolute probability that they will experience a CVD event in a given time period — than to isolated individual risk factors or relative risks.3,4
Accurate assessment of absolute CVD risk applies quantitative data on multiple factors that influence risk, including smoking status, blood pressure (BP), blood lipid levels, and diabetes status, to a person’s age- and sex-specific background level of absolute risk.3 Assessment that is based only on individual risk factors (eg, considering cholesterol levels alone) and does not use tools that allow quantification of overall absolute CVD risk leads to substantial misclassification, a general underestimation of risk, and under- and overtreatment.5 Quantitative absolute risk models that assess risk and guide management are central to the primary prevention of CVD, nationally and internationally.6–9
Population-level data on absolute CVD risk have the potential to inform programs, policy and planning, including those associated with implementing large scale treatment strategies according to absolute CVD risk.10 However, population-level data on absolute CVD risk are not available for most countries, including Australia.
Our investigation aimed to quantify absolute CVD risk in the Australian adult population, as well as treatment with BP- and lipid-lowering medications, using data from a representative health survey. It focused on individuals aged 45–74 years, the age group for which most risk calculators have been validated, and with the greatest population burden of premature CVD.
Methods
Study population
The study population comprised 9564 participants from the Australian Bureau of Statistics (ABS) Australian Health Survey11 aged 18 years or over who provided biomedical data for the National Health Measures Survey (NHMS) between March 2011 and September 2012. Details of the Australian Health Survey and NHMS are provided in the Appendix. Of the 30 329 respondents eligible to participate in the NHMS (ie, Australian Health Survey participants aged 5 years and over), 11 246 (37.1%) did so (46.5% of those aged 45–74 years).
Data and variables
Data on sociodemographic and health-related factors, including prior CVD, medical history and health behaviours, were provided by self-report at the home-based interview for the Australian Health Survey. Height, weight, waist circumference, and systolic and diastolic BP were measured directly, fasting blood samples taken and assayed, and a medications review conducted, using standard methods. Details of these methods and the derived variables (eg, diabetes status) are included in the Appendix, in reference and in Box 1.
Absolute risk of a primary CVD event
The absolute risk of a primary CVD event over the next 5 years for a participant without prior CVD was estimated by applying the Australian National Vascular Disease Prevention Alliance (NVDPA) risk assessment and risk management algorithm, which includes the Framingham CVD risk equation;7,11–13 see the Appendix for details. The absolute risk of a primary CVD event over the next 5 years was categorised using the cut-points: low (< 10%); moderate (10–15%); high (> 15%).7
Statistical methods
All analyses were conducted by staff at the ABS, in collaboration with the authors and using ABS statistical programs, in accordance with the Australian Census and Statistics Act 1905 (Cth), supplemented by calculations by the authors, based on these analyses.
The proportions of people with and without prior CVD were calculated for the entire population aged 18 years or more, as well as by sex and age group. Among those without prior CVD, the distributions of the risk factors contributing to the CVD risk algorithm were estimated for those aged 45 years or more.
The proportions of those aged 18 years or more with low, moderate and high absolute CVD risk were calculated for those without prior CVD and presented according to age group and sex. In those aged 45–74 years, the proportions of those with selected health characteristics and risk factors and the proportions receiving BP- and/or lipid-lowering medications were estimated according to prior CVD and absolute primary CVD risk.
Weights were applied to all prevalence estimates to account for the sampling strategy and non-response (see Appendix). The numbers of individuals in Australia with different CVD risk factors, absolute risk levels, and treatment profiles were estimated by applying the weighted proportions to Australian general population data.11 Standard errors were calculated, taking into account variability due to sampling and to random adjustment, as were 95% confidence intervals (CIs).
Ethics approval
Ethics approval for NHMS data collection was provided by the Australian Government Department of Health Human Research Ethics Committee (reference 2/2011). Additional approval was granted by the Australian National University Human Research Ethics Committee (reference 2014/208).
Results
Respondents for whom data were missing about previous CVD or any components of the risk assessment algorithm, including factors in the Framingham CVD risk equation, were excluded from the analyses; 1059 participants were excluded by these criteria. We therefore analysed the data for 8505 participants aged 18 years or more (3828 men and 4677 women), of whom 4844 were aged 45–74 years (2210 men, 2634 women). The Appendix includes the separate results for men and women.
Prior CVD and absolute risk of a primary CVD event
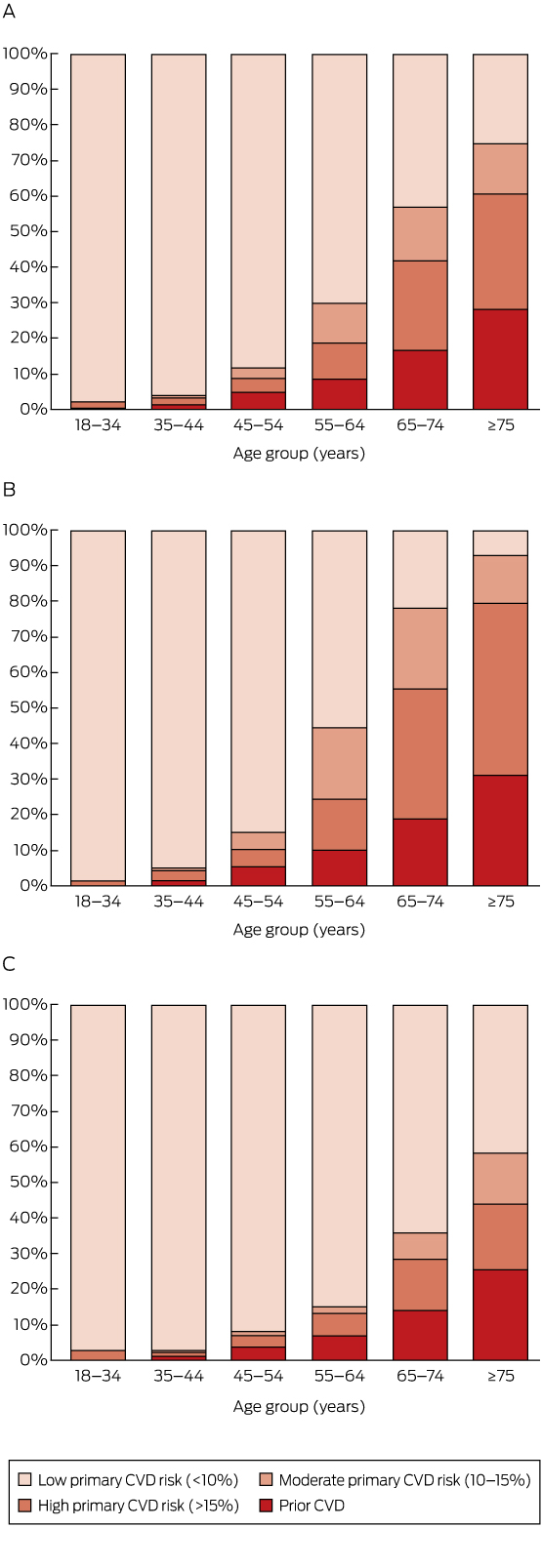
Overall, 6.2% of those aged 18 years or more (corresponding to an estimated 1 071 000 adults in Australia), including 8.7% (95% CI, 7.8–9.6%) of those aged 45–74 years (an estimated 634 000 adults), were classified as having prior CVD (Box 2).
A total of 8.2% of those aged 18 years or more (an estimated 1 412 000 adults) were at high absolute risk of a primary CVD event (Box 2). The proportion at high primary risk was 1.9% in those aged 18–44 years, and increased with age (Box 2, Box 3). Major risk factors were often present in those aged 45–74 years without prior CVD (Box 1, Appendix).
Among those aged 45–74 years, 71.5% (95% CI, 70.1–72.9%; an estimated 5 209 000 people) were at low risk of a primary CVD event, 8.6% (95% CI, 7.4–9.8%; an estimated 625 000 people) were at moderate primary risk, and 11.2% (95% CI, 10.2–12.2%; an estimated 811 000 people) were at high primary risk (Box 2).
Combining those with prior CVD and those at high risk of a primary event, an estimated 19.9% (95% CI, 18.5–21.3%) of people in Australia aged 45–74 years had a high 5-year risk of a CVD event (an estimated 1 445 000 people), including 25.8% (95% CI, 23.4–28.2%) of men (an estimated 925 000 people) and 14.2% (95% CI, 12.6–15.8%) of women (an estimated 522 000 people) in this age bracket (Box 2, Appendix). As would be expected, risk was related to major CVD risk factors (Appendix, Table S2).
BP- and lipid-lowering medications
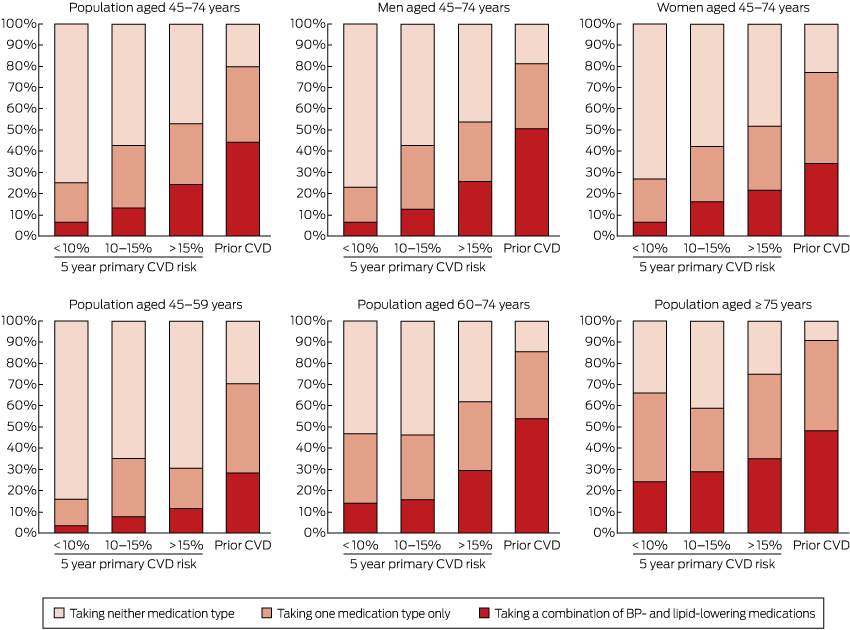
Use of BP- and lipid-lowering medications was substantially more common in those with prior CVD and in those at higher primary risk than in those at lower risk (Box 4, Box 5; Appendix, Table S3). In those aged 45–74 years with prior CVD, 44.2% were receiving both BP- and lipid-lowering medications (an estimated 280 000 people), 35.4% were receiving only one of these medication types (an estimated 225 000 people), and 20.4% were receiving neither (an estimated 129 000 people) (Box 4).
Among people aged 45–74 years who did not have prior CVD and were at high absolute risk of a primary event, 24.3% were receiving both BP- and lipid-lowering medications (an estimated 197 000 people), 28.7% were receiving only one of these medication types (an estimated 233 000 people), and 47.1% were receiving neither (an estimated 382 000 people) (Box 4). Corresponding figures for those at moderate primary CVD risk were 13.2% (an estimated 82 000 people); 29.5% (an estimated 185 000 people); and 57.3% (an estimated 358 000 people). Of those at low primary CVD risk, 6.6% were receiving both BP- and lipid-lowering medications (an estimated 346 000 people), 18.6% were receiving only one of these medication types (an estimated 966 000 people), and 74.8% were receiving neither (an estimated 3 896 000 people) (Box 4).
Discussion
An estimated one-fifth of the Australian population aged 45–74 years, or about 1.4 million people, had a greater than 15% absolute risk of a CVD event in the next 5 years. This was made up of 11% of individuals aged 45–74 years (about 800 000 people) with a greater than 15% probability of a primary CVD event in the next 5 years, and 9% (about 630 000 people) with existing CVD. Although the risk assessment tools used have not generally been validated outside this age range, our data also suggest that around 2.6% of the population aged 18–44 years (about 230 000 people) and 60.6% of those aged 75 years or more (about 850 000 people) were at high absolute risk of a future CVD event. Levels of absolute CVD risk were higher for men than for women; risk also increased markedly with age.
The Australian NVDPA algorithm uses global CVD (which encompasses coronary heart disease, cerebrovascular disease, peripheral vascular disease and heart failure) as its predicted outcome.14 For more than a decade, Australian national guidelines have recommended lipid-lowering therapies for those at high absolute CVD risk, and consideration of absolute CVD risk in the management of hypertension.15,16 The 2012 NVDPA guidelines recommend combination treatment with BP- and lipid-lowering medications unless contraindicated or clinically inappropriate, together with lifestyle advice, for those with a 5-year primary CVD risk of more than 15%; it advises considering combination treatment for those at moderate risk (5-year primary CVD risk of 10–15%) if 3 to 6 months of lifestyle intervention does not reduce risk sufficiently, or if certain risk factors are present, such as a family history of premature CVD.7 Recently, United Kingdom and United States guidelines have changed from recommending lipid-lowering treatment for those with a 10-year CVD risk of 20% or greater (roughly equivalent to the Australian moderate and high risk categories) to recommending it for individuals with 10-year CVD risks of 10% (UK)9 or 7.5% (US)8 or greater.
We were unable to identify any previous studies that had profiled absolute global CVD risk for a national population, integrating representative information on the absolute risk of primary and secondary CVD events, and treatment with BP- and lipid-lowering medications. A recent article reported that 15.5% of the US population aged 20–79 years was at high absolute risk of a future coronary heart disease event (rather than global CVD event) within 10 years according to the US Adult Treatment Panel (ATP) III guidelines.17 Although CVD absolute risk is likely to vary by population and over time, these US results on coronary heart disease are comparable with our findings that 6.2% of those aged 18 years or more have prior CVD of any kind, and that 13.0% have at least a 10% primary risk of global CVD over the next 5 years.17,18
About half of those with prior CVD aged 45–74 years and one-quarter of those with a 5-year primary CVD risk above 15% were receiving both BP- and lipid-lowering medications, compared with one in 15 of those at low primary risk. This indicates that these medications were, to some extent, being targeted. At the same time, large opportunities for improvement in treatment are apparent, especially in the younger age group: 76% of individuals aged 45–74 years and 88% of individuals aged 45–59 years with a 5-year primary CVD risk greater than 15% were not receiving combination BP- and lipid-lowering treatment.
Applying our estimates to the general population in 2011–12, an estimated 510 000 individuals aged 45–74 years in Australia were at high risk of primary CVD or had prior CVD and were receiving neither BP-lowering nor lipid-lowering medications; a further 460 000 in these groups were receiving only one of the two medication types. This suggests that up to 970 000 people, or 13% of the Australian population aged 45–74 years, have a 5-year risk of a CVD event greater than 15% and are not receiving currently recommended combination BP- and lipid-lowering therapies. A further 620 000 people are at moderate absolute risk of CVD, with about 540 000 not receiving combination BP- and lipid-lowering therapies.
BP- and lipid-lowering treatments were the focus of our analyses because they are the main treatments for preventing CVD events, and are indicated in both primary and secondary prevention. Additional medications, including anti-platelet agents, are recommended for individuals with existing CVD. The treatment gaps we have identified are therefore likely to be underestimates.
The general findings that BP- and lipid-lowering treatment was more common among those at high risk than in those at low risk, and that there were considerable gaps in treatment, are broadly consistent with findings from earlier clinical and non-representative studies, despite the generally higher levels of CVD risk in these samples.19,20 These studies found that 40–75% of patients with existing CVD were receiving both BP- and lipid-lowering therapies,21–23 consistent with the 44% combined treatment rate we found. Also consistent are primary care data from Australia and New Zealand which indicate that about one-quarter of those with a 5-year primary risk of 15% or more are taking combination BP- and lipid-lowering treatments.19,20,22
Our findings concern the general population, a substantial proportion of whom are receiving treatment likely to influence their CVD risk and risk factors. The Framingham Risk Equation is aimed at treatment-naive individuals and will tend to underestimate risk in those receiving treatment. Ideally, absolute CVD risk should be assessed before commencing treatment; however, this is not practical for a population-based sample of this type. It is not possible to reliably ascertain the underlying prior absolute CVD risk status of those who are being treated with BP- and/or lipid-lowering medications and who are assessed as being at low or moderate absolute CVD risk, as they may have moved to a lower risk category because of changes in BP and/or blood lipid profiles. Further, treatment with BP- and lipid-lowering medication is still recommended in some contexts for individuals with abnormalities in single risk factors. Consequently, while lipid-lowering treatments are not recommended for those at low CVD risk, data from our study are relatively uninformative with regard to potential overtreatment in this group.
The gap between current guideline-recommended treatment and the use of BP- and lipid-lowering medications we describe is likely to be related to a number of factors, including the extent of absolute risk-based CVD assessment, appropriate prescribing and uptake, and continuation of treatment. In Australia, CVD risk assessment is recommended from age 45 for the general population and from age 35 for people of Aboriginal and/or Torres Strait Islander background.7 The fact that more than 97% of individuals aged 18–44 years in our study were estimated to be at low primary CVD risk supports the current age cut-off for assessment. It is not known what proportion of the general population undergoes guideline-based absolute CVD risk assessment. Recent evidence indicates that less than half of the eligible individuals attending primary care have had a documented quantitative CVD risk assessment.20,22,23 Although BP- and lipid-lowering medications are generally well tolerated and relatively safe, it is likely that their not being used is, in some cases, the result of contraindications, adverse drug events, a lack of prescription, cost, or their being declined by the patient. Further, international summary estimates of continuation with BP-lowering medications are 42–61% and 62–79% for primary and secondary prevention of coronary heart disease respectively; corresponding figures for statins are 57% and 76%.24
The NHMS assessed CVD risk and medication using inclusive home interview-based sampling and high quality methods. Despite its large size, particularly in comparison with other representative surveys, numbers were limited and confidence intervals were wide in some subgroups. There were insufficient numbers of Aboriginal and Torres Strait Islander participants to quantify risk in this group; relevant analyses would be beneficial when appropriate data become available. The study response rate was 46.5% in the target population (those aged 45–74 years) and the study methods produced estimates representative of the general non-institutionalised Australian population. The NHMS used validated data collection tools that have inherent limitations, including a range of measures based on self-report (such as smoking status, alcohol consumption, and previous CVD). Data were lacking for some measures incorporated into the NVDPA algorithm, including familial hypercholesterolaemia and persistent proteinuria. These limitations and the under-inclusion of institutionalised participants (who are more likely to be older and to have a greater illness burden) mean that absolute CVD risk may have been underestimated, particularly in those over 74 years of age.
Implications
Our results highlight the ongoing efforts and the major opportunities for reducing the frequency of CVD events. Implementation of large scale CVD risk assessment and treatment based on absolute risk is considered to be one of the most cost-effective interventions.25 We estimate that about 870 000 individuals aged 45–74 years at moderate to high absolute risk of CVD are receiving neither BP- nor lipid-lowering therapies. Broadly speaking, lowering systolic BP by around 8 mmHg in this group would prevent an estimated 20 to 37 cardiovascular events per 1000 treated;4 an estimated 31 to 61 events would be prevented for each 1 mmol/L reduction in low-density lipoprotein cholesterol levels.26 While detailed modelling is required for accurate quantification, these findings indicate that more extensive treatment of this single risk group alone could prevent tens of thousands of CVD events in Australia.
Box 1 –
Estimated distribution of cardiovascular disease (CVD) risk factors included in the National Vascular Disease Prevention Alliance algorithm for the Australian-population without prior CVD, by age group
|
Characteristic
|
Age group (years)
|
|
45–54
|
55–64
|
65–74
|
≥ 75
|
Total (≥ 45)
|
|
|
Total number
|
|
|
|
|
|
|
Smoking status
|
|
|
|
|
|
|
Never smoked
|
48.1%
|
48.7%
|
49.6%
|
48.9%
|
48.7%
|
|
Ex-smoker
|
37.8%
|
38.6%
|
43.4%
|
48.4%
|
40.4%
|
|
Current smoker
|
14.1%
|
12.7%
|
7.0%
|
2.6%
|
11.0%
|
|
Systolic blood pressure
|
|
|
|
|
|
|
< 120 mmHg
|
49.3%
|
33.3%
|
21.1%
|
16.6%
|
35.3%
|
|
120–139 mmHg
|
35.9%
|
40.1%
|
40.9%
|
40.0%
|
38.6%
|
|
140–179 mmHg
|
14.5%
|
25.6%
|
35.8%
|
37.6%
|
24.6%
|
|
≥ 180 mmHg
|
0.2%
|
1.0%
|
2.2%
|
5.7%
|
1.5%
|
|
Diastolic blood pressure
|
|
|
|
|
|
|
< 90 mmHg
|
83.0%
|
84.3%
|
86.8%
|
91.3%
|
85.1%
|
|
90–109 mmHg
|
16.7%
|
15.3%
|
12.5%
|
8.0%
|
14.5%
|
|
≥ 110 mmHg
|
0.3%
|
0.4%
|
0.7%
|
0.7%
|
0.4%
|
|
Low density lipoprotein cholesterol
|
|
|
|
|
|
|
< 2.0 mmol/L
|
2.0%
|
4.0%
|
9.1%
|
12.4%
|
5.2%
|
|
2.0–3.5 mmol/L
|
52.5%
|
47.6%
|
51.7%
|
59.8%
|
51.6%
|
|
> 3.5 mmol/L
|
45.4%
|
48.4%
|
39.2%
|
27.8%
|
43.2%
|
|
High density lipoprotein cholesterol
|
|
|
|
|
|
|
≥ 1.0 mmol/L
|
88.7%
|
89.8%
|
89.7%
|
91.5%
|
89.6%
|
|
< 1.0 mmol/L
|
11.3%
|
10.2%
|
10.3%
|
8.5%
|
10.4%
|
|
Total cholesterol
|
|
|
|
|
|
|
< 4.0 mmol/L
|
4.7%
|
6.2%
|
10.8%
|
15.3%
|
7.6%
|
|
4.0–7.5 mmol/L
|
92.8%
|
91.5%
|
87.0%
|
83.3%
|
90.2%
|
|
> 7.5 mmol/L
|
2.5%
|
2.3%
|
2.2%
|
1.5%
|
2.2%
|
|
Total cholesterol:high density lipoprotein cholesterol ratio
|
|
|
|
|
|
|
< 4.5
|
62.3%
|
67.8%
|
73.7%
|
79.7%
|
68.2%
|
|
4.5–5.9
|
28.3%
|
24.1%
|
20.1%
|
16.5%
|
24.0%
|
|
≥ 6
|
9.4%
|
8.2%
|
6.2%
|
3.7%
|
7.7%
|
|
Diabetes
|
4.7%
|
8.1%
|
14.5%
|
11.2%
|
8.4%
|
|
Diabetes with microalbuminuria
|
0.8%
|
1.4%
|
4.5%
|
4.0%
|
2.1%
|
|
Moderate to severe chronic kidney disease
|
0.1%
|
0.4%
|
1.7%
|
6.8%
|
1.3%
|
|
|
|
Box 2 –
Estimated proportions and numbers of individuals in the Australian population with prior cardiovascular disease (CVD), and among those without prior CVD with low, moderate or high absolute 5-year risk of a primary CVD event, by age group and sex
|
Age group and sex
|
No prior CVD
|
Prior CVD
|
|
Absolute CVD risk category
|
|
|
|
Low (< 10%)
|
Moderate (10–15%)
|
High (> 15%)
|
|
|
|
% [95% CI]
|
N*
|
% [95% CI]
|
N*
|
% [95% CI]
|
N*
|
% [95% CI]
|
N*
|
|
|
Total population
|
|
18–34 years
|
97.8 [96.6–99.0]
|
5271
|
0.0 [0.0–0.0]
|
0
|
1.8 [0.8–2.8]
|
98
|
0.4 [0.0–0.8]
|
19
|
|
35–44 years
|
96.3 [95.2–97.4]
|
3042
|
0.3 [0.0–0.6]
|
9
|
2.1 [1.3–2.9]
|
68
|
1.3 [0.5–2.1]
|
41
|
|
45–54 years
|
88.4 [86.5–90.3]
|
2685
|
3.0 [1.7–4.3]
|
90
|
4.0 [2.7–5.3]
|
121
|
4.6 [3.3–5.9]
|
139
|
|
55–64 years
|
70.3 [67.7–72.9]
|
1800
|
11.0 [8.9–13.1]
|
281
|
10.2 [8.3–12.1]
|
263
|
8.5 [6.7–10.3]
|
218
|
|
65–74 years
|
43.1 [39.7–46.5]
|
724
|
15.1 [12.7–17.5]
|
254
|
25.4 [22.5–28.3]
|
427
|
16.4 [13.9–18.9]
|
277
|
|
≥ 75 years
|
25.6 [20.4–30.8]
|
359
|
13.8 [10.8–16.8]
|
194
|
32.4 [27.5–37.3]
|
456
|
28.2 [23.1–33.3]
|
396
|
|
Total
|
80.8 [80.0–81.6]
|
13 923
|
4.8 [4.3–5.3]
|
828
|
8.2 [7.5–8.9]
|
1412
|
6.2 [5.6–6.8]
|
1071
|
|
Men
|
|
18–34 years
|
98.5 [97.3–99.7]
|
2693
|
0.0 [0.0–0.0]
|
0
|
1.4 [0.3–2.5]
|
40
|
0.0 [0.0–0.0]
|
0
|
|
35–44 years
|
95.1 [93.2–97.0]
|
1492
|
0.4 [0.0–0.9]
|
6
|
3.1 [1.4–4.8]
|
48
|
1.4 [0.3–2.5]
|
23
|
|
45–54 years
|
85.0 [81.7–88.3]
|
1278
|
4.9 [2.5–7.3]
|
74
|
4.8 [2.6–7.0]
|
72
|
5.4 [3.1–7.7]
|
81
|
|
55–64 years
|
55.5 [51.1–59.9]
|
706
|
20.1 [16.0–24.2]
|
256
|
14.3 [11.4–17.2]
|
183
|
10.0 [7.1–12.9]
|
128
|
|
65–74 years
|
21.9 [17.6–26.2]
|
182
|
22.7 [18.8–26.6]
|
188
|
36.6 [31.4–41.8]
|
305
|
18.8 [14.7–22.9]
|
156
|
|
≥ 75 years
|
7.1 [3.0–11.2]
|
41
|
13.3 [9.3–17.3]
|
78
|
48.5 [41.5–55.5]
|
285
|
31.1 [24.6–37.6]
|
183
|
|
Total
|
74.8 [73.5–76.1]
|
6361
|
7.2 [6.2–8.2]
|
615
|
11.1 [10.1–12.1]
|
943
|
6.8 [5.9–7.7]
|
579
|
|
Women
|
|
18–34 years
|
97.1 [95.2–99.0]
|
2577
|
0.0 [0.0–0.0]
|
0
|
2.2 [0.5–3.9]
|
59
|
0.7 [0.0–1.5]
|
19
|
|
35–44 years
|
97.5 [96.2–98.8]
|
1552
|
0.2 [0.0–0.6]
|
3
|
1.2 [0.5–1.9]
|
19
|
1.2 [0.2–2.2]
|
19
|
|
45–54 years
|
91.8 [89.5–94.1]
|
1405
|
1.1 [0.2–2.0]
|
18
|
3.3 [1.8–4.8]
|
51
|
3.8 [2.3–5.3]
|
59
|
|
55–64 years
|
84.8 [81.6–88.0]
|
1093
|
1.9 [1.1–2.7]
|
26
|
6.2 [4.0–8.4]
|
80
|
7.0 [5.1–8.9]
|
90
|
|
65–74 years
|
64.0 [59.7–68.3]
|
544
|
7.5 [5.1–9.9]
|
64
|
14.4 [11.8–17.0]
|
122
|
14.1 [10.9–17.3]
|
120
|
|
≥ 75 years
|
41.6 [33.3–49.9]
|
340
|
14.3 [9.9–18.7]
|
117
|
18.5 [12.1–24.9]
|
151
|
25.6 [18.1–33.1]
|
210
|
|
Total
|
86.6 [85.6–87.6]
|
7570
|
2.4 [1.9–2.9]
|
210
|
5.3 [4.4–6.2]
|
466
|
5.6 [4.8–6.4]
|
490
|
|
|
* N = estimated number, in thousands, of persons in each category in Australia. Weighting and missing values mean that numbers do not always sum to totals.
|
Box 3 –
Estimated distribution of prior cardiovascular disease (CVD) and absolute risk of a primary CVD event over the next 5 years by age group, in the total Australian population aged 18 years or more (A) and for men (B) and women (C) separately
Box 4 –
Estimated proportions and numbers of individuals aged 45 or more in the Australian who were receiving blood pressure (BP)-lowering* and/or lipid-lowering† medications, according to cardiovascular disease (CVD) risk
|
|
No prior CVD
|
Prior CVD
|
Total
|
|
Absolute primary CVD risk category
|
|
|
|
|
|
Low (<10%)
|
Moderate (10-15%)
|
High (>15%)
|
|
|
|
|
|
% [95% CI]
|
N‡
|
% [95% CI]
|
N‡
|
% [95% CI]
|
N‡
|
% [95% CI]
|
N‡
|
% [95% CI]
|
N‡
|
|
|
Population aged 45–74 years
|
|
Lipid-lowering medication
|
13.7 [11.4–16.0]
|
714
|
20.1 [13.2–27.0]
|
126
|
32.5 [25.9–39.1]
|
265
|
55.7 [47.0–64.4]
|
354
|
19.8 [17.8–21.8]
|
1438
|
|
No lipid-lowering medication
|
86.3 [83.9–88.7]
|
4494
|
79.9 [73.0–86.8]
|
499
|
67.5 [60.9–74.1]
|
548
|
44.3 [35.5–53.1]
|
281
|
80.2 [78.2–82.2]
|
5842
|
|
BP-lowering medication
|
18.1 [15.9–20.3]
|
945
|
35.8 [27.1–44.5]
|
223
|
44.7 [37.7–51.7]
|
362
|
68.1 [60.5–75.7]
|
432
|
26.6 [24.3–28.9]
|
1940
|
|
No BP-lowering medication
|
81.9 [79.7–84.1]
|
4262
|
64.2 [55.5–72.9]
|
401
|
55.3 [48.3–62.3]
|
449
|
31.9 [24.3–39.5]
|
202
|
73.4 [71.1–75.7]
|
5340
|
|
BP- and lipid-lowering medication
|
6.6 [5.1–8.1]
|
346
|
13.2 [7.7–18.7]
|
82
|
24.3 [18.3–30.3]
|
197
|
44.2 [36.8–51.6]
|
280
|
12.2 [10.6–13.8]
|
889
|
|
Taking one medication only
|
18.6 [16.2–21.0]
|
966
|
29.5 [21.8–37.2]
|
185
|
28.7 [22.7–34.7]
|
233
|
35.4 [27.8–43.0]
|
225
|
22.0 [20.1–23.9]
|
1599
|
|
Taking neither medication
|
74.8 [72.2–77.4]
|
3896
|
57.3 [48.4–66.2]
|
358
|
47.1 [39.9–54.3]
|
382
|
20.4 [13.9–26.9]
|
129
|
65.8 [63.3–68.3]
|
4792
|
|
Population aged ≥ 75 years
|
|
Lipid-lowering medication
|
39.3 [25.5–53.1]
|
142
|
30.8 [16.7–44.9]
|
60
|
43.0 [30.8–55.2]
|
196
|
59.7 [45.9–73.5]
|
236
|
44.1 [36.6–51.6]
|
621
|
|
No lipid-lowering medication
|
60.7 [46.9–74.5]
|
219
|
69.2 [55.1–83.3]
|
134
|
57.0 [44.8–69.2]
|
259
|
40.3 [26.5–54.1]
|
160
|
55.9 [48.4–63.4]
|
785
|
|
BP-lowering medication
|
51.0 [35.3–66.7]
|
184
|
56.9 [38.9–74.9]
|
110
|
66.6 [58.6–74.6]
|
304
|
79.1 [69.5–88.7]
|
313
|
63.9 [58.0–69.8]
|
899
|
|
No BP-lowering medication
|
49.0 [33.3–64.7]
|
176
|
43.1 [25.2–61.0]
|
84
|
33.4 [25.3–41.5]
|
152
|
20.9 [11.3–30.5]
|
83
|
36.1 [30.2–42.0]
|
507
|
|
BP- and lipid-lowering medication
|
24.2 [12.9–35.5]
|
87
|
28.9 [14.5–43.3]
|
56
|
34.9 [24.2–45.6]
|
158
|
48.3 [35.0–61.6]
|
192
|
34.3 [27.8–40.8]
|
482
|
|
Taking one medication only
|
41.9 [28.8–55.0]
|
151
|
30.0 [15.9–44.1]
|
59
|
39.8 [28.4–51.2]
|
181
|
42.3 [29.6–55.0]
|
167
|
39.4 [32.2–46.6]
|
555
|
|
Taking neither medication
|
33.9 [19.7–48.1]
|
121
|
41.2 [23.6–58.8]
|
81
|
25.3 [16.2–34.4]
|
115
|
9.4 [2.9–15.9]
|
37
|
26.3 [19.7–32.9]
|
369
|
|
|
* Hypertension medications includes Anatomic Therapeutic Chemical Classification C02 (antihypertensives), C03 (diuretics), C07 (beta blocking agents), C08 (calcium channel blockers) and C09 (agents acting on the renin–angiotensin system). † Lipid-lowering medications include Anatomic Therapeutic Chemical Classification C10 (lipid modifying agents, plain and combinations. ‡ N = estimated number, in thousands, of persons in each category in Australia. Weighting and missing values mean that numbers do not always sum to totals.
|
Box 5 –
Estimated distribution of Australians receiving blood pressure (BP)-lowering* and/or lipid-lowering† medications, by absolute risk of primary cardiovascular disease (CVD) event or prior CVD, by sex and age group

 more_vert
more_vert