In most industrialised countries, including Australia, the incidence of oesophageal adenocarcinoma has increased fivefold in the past 40 years.1 Almost all of these cancers arise from underlying Barrett’s oesophagus,2 a condition described by Australian-born Norman Barrett in 19573 in which the normal oesophageal squamous epithelium is partially replaced by an intestinal metaplastic columnar epithelium. This narrative review discusses the epidemiology of Barrett’s oesophagus and its relationship to cancer, considers recent developments around screening and surveillance, and briefly reviews the management of dysplasia and early adenocarcinoma arising in Barrett’s oesophagus. It is based on comprehensive Australian guidelines recently published by Cancer Council Australia (http://wiki.cancer.org.au/australia/Guidelines: Barrett%27s).4
Definition
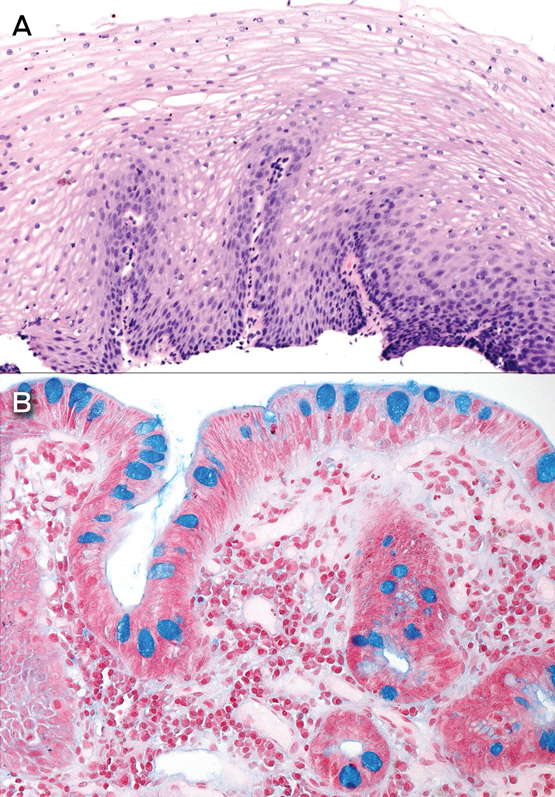
In the Australian guidelines (as in most other international guidelines), a diagnosis of Barrett’s oesophagus requires two components: first, endoscopic evidence of a salmon-pink coloured columnar epithelium extending above the gastro-oesophageal junction and partially replacing the normal tubular oesophageal squamous epithelium; and second, biopsies from the oesophageal columnar epithelium showing evidence of intestinal metaplasia, with the presence of mucin-containing goblet cells (Box 1).4–6 Under Australian guidelines, patients with a columnar-lined oesophagus on endoscopy but no evidence of intestinal metaplasia on biopsy do not meet the definition for Barrett’s oesophagus; the significance of this finding is uncertain, and we discuss the management of such patients below.
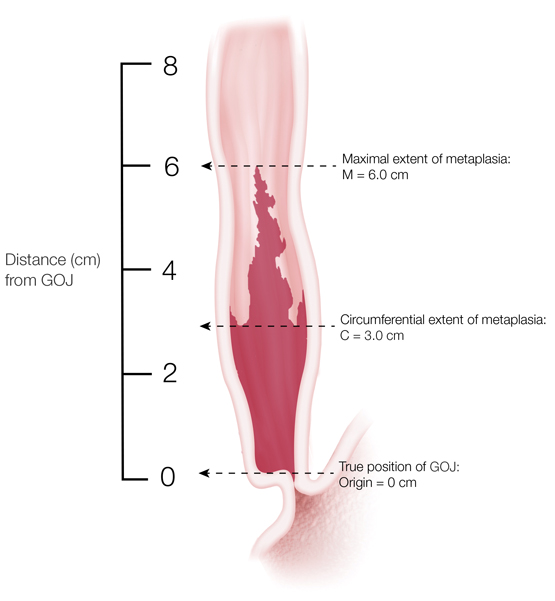
The length of the columnar epithelium at endoscopy is described using the Prague C (circumferential length) and M (maximal length) criteria (Box 2).7 Barrett’s oesophagus is defined as long segment when maximal segment length is ≥ 3 cm and as short segment when maximal length is < 3 cm.
Prevalence
Because Barrett’s oesophagus is asymptomatic and requires endoscopic examination and histological confirmation to establish the diagnosis, estimates of prevalence in unselected populations are scarce. The arguably best data were derived from a sample of 1000 Swedish residents recruited at random from the community who underwent upper gastrointestinal endoscopy, of whom 16 were identified with Barrett’s oesophagus (5 long segment, 11 short segment).8 Well conducted surveys in comparable populations (the United States and Europe) suggest community prevalence < 5%, with estimates converging around 2%; Australian data are limited to studies of patients referred for endoscopic investigation of symptoms.9–11 There is evidence that Australian detection rates have increased recently, with higher proportions of patients who undergo upper gastrointestinal endoscopy being diagnosed with Barrett’s oesophagus in consecutive surveys (rising from 0.3% in 1990 to 1.9% in 2002).10
Risk factors
Pooled analyses and meta-analyses of high quality epidemiological studies have consistently identified age, male sex, gastro-oesophageal reflux, central obesity and smoking as risk factors for Barrett’s oesophagus. In most populations, Barrett’s oesophagus is twice as common in men as in women,12 and prevalence rises with age.13
The longstanding clinical association between Barrett’s oesophagus and acid regurgitation or heartburn has been confirmed in research studies; a recent meta-analysis concluded that symptoms of gastro-oesophageal reflux increased the risks of long segment Barrett’s oesophagus more than fivefold.14 In addition, factors that promote reflux, such as hiatal hernia, are also observed more frequently in patients with Barrett’s oesophagus than among endoscopy controls with non-erosive reflux disease.15
While obesity is an established risk factor for oesophageal adenocarcinoma, epidemiologic studies have reported inconsistent associations between body mass index and Barrett’s oesophagus.16 However, studies measuring abdominal obesity (eg, waist circumference, waist–hip ratio) have identified two-to-threefold higher risks for high versus low waist circumference.16 Strong evidence of a likely causal association between obesity and Barrett’s oesophagus came from a Mendelian randomisation analysis, in which investigators demonstrated that people with a strong genetic propensity to develop obesity have significantly higher risks of Barrett’s oesophagus than those with a weak genetic propensity to obesity.17 The mechanisms remain speculative, but include mechanical (increased pressure on the lower oesophageal sphincter promoting reflux), metabolic and hormonal pathways. Importantly, the association between abdominal obesity and Barrett’s oesophagus is observed in people with and without reflux symptoms, indicating that mechanical reflux does not explain the whole effect.18 Metabolic factors are strongly implicated, with recent investigations reporting positive associations with markers of the metabolic syndrome, including insulin resistance19 and high serum concentrations of leptin.20–22 Increasingly, it seems likely that male–female differences in fat deposition and metabolism may account for some of the observed sex-specific differences in the prevalence of Barrett’s oesophagus.23
Many other lifestyle exposures have been assessed as possible risk factors for Barrett’s oesophagus, two of which have been consistently implicated. Smoking increases the risk of the condition by about 50%24,25, whereas past infection with Helicobacter pylori reduces risk by about 50%.26,27 Previously, it was hypothesised that H. pylori infection inhibits gastric acid production and thus reduces acid-associated damage.27 Recent studies suggest that, in Western populations, H. pylori infection occurs predominantly in the antrum and likely reduces the risk of Barrett’s oesophagus by disrupting ghrelin and leptin pathways.28,29
Aside from the factors described above, no others have been consistently associated with the disease. Thus, despite considerable investigation, there is no evidence that alcohol is a risk factor.30–33 Similarly, several well conducted case–control studies34,35 have investigated the role of aspirin and other non-steroidal anti-inflammatory drugs, based on strong and consistent inverse associations with oesophageal adenocarcinoma in observational and experimental studies. However, there is no evidence that this class of drugs alters a person’s risk of Barrett’s oesophagus. Relatively few studies have examined dietary factors and no conclusions can be drawn.
In the past 5 years, several large scale genome-wide association studies have identified a number of single nucleotide polymorphisms significantly associated with Barrett’s oesophagus.36 Moreover, there appears to be considerable genetic overlap between patients with Barrett’s oesophagus and patients with oesophageal adenocarcinoma, lending weight to the notion that these two conditions share similar causal origins.37,38 This is a rapidly moving field, but the clinical utility of these findings remains unknown.
Progression to cancer
Clinical interest in Barrett’s oesophagus stems largely from the concern that the condition is a precursor or risk marker for adenocarcinoma of the oesophagus. Most cases of oesophageal adenocarcinoma arise from underlying Barrett’s metaplasia in which there is a histological progression over time from low grade dysplasia (LGD) to high grade dysplasia (HGD) and subsequent intramucosal and invasive carcinoma (metaplasia–dysplasia–carcinoma sequence). Early oesophageal adenocarcinoma refers to invasion of the carcinoma beyond the basement membrane into the lamina propria (T1a on the current tumour–node–metastasis staging system) or superficial submucosa (T1b), but no deeper. Early oesophageal adenocarcinoma represents 6–12% of patients presenting with oesophageal cancer.39,40
The key question relates to the rate at which patients diagnosed with Barrett’s oesophagus progress to cancer. Early studies, largely conducted in tertiary referral centres, suggested rates as high as 1–2 per 100 patients per year. Since the year 2000, a number of large, population-based, record linkage studies have observed considerably lower progression rates for patients with uncomplicated Barrett’s oesophagus, converging at around 1–3 per 1000 patients per year (an order of magnitude lower than earlier reports).41–45 The risk of progression is greater in those with dysplasia46 and those with long segment Barrett’s oesophagus.47
Considerable uncertainty remains about progression rates among Barrett’s oesophagus patients with LGD. In the community, LGD patients progress to HGD or cancer at a rate of about 1.5% per annum, whereas a recent European academic centre-based study reported much higher progression rates to HGD or cancer (about 13% per annum).42,48 The explanation appears to be that patients attending academic centres are reviewed by multiple expert gastrointestinal pathologists, and up to 75% of community referral LGD patients are downstaged to non-dysplastic Barrett’s oesophagus following expert review. Among the downstaged patients, progression rates to HGD or cancer of about 0.5% per year have been observed,48 similar to those reported from community-based studies.42 Studies from other academic centres of progression rates of patients with expert-confirmed LGD are awaited.
Little is known about lifestyle factors that increase or decrease the rate of progression to cancer. The literature underpinning this area is limited in scope and challenged by methodological issues such as small sample sizes, losses to follow-up, possible selection bias and confounding. Notwithstanding these limitations, it would seem that men with Barrett’s oesophagus progress to cancer at about twice the rate of women,46,49 and smokers progress at twice the rate of non-smokers.50,51 While prospective observational studies suggest that non-steroidal anti-inflammatory drugs,52,53 proton pump inhibitors54 and statins52,55 might retard progression to cancer, to date there are no randomised trials to support such conclusions and caution is warranted. Clinical factors associated with high rates of progression include longer segment length,45,46,50,52 and the presence of nodules,56 ulceration57 and strictures57 on endoscopy.
Screening
Screening is the process of identifying new cases of disease in an unselected population. Endoscopic screening for Barrett’s oesophagus in an unselected population with gastro-oesophageal reflux symptoms is not recommended, as it is not cost-effective. Focused endoscopic screening programs in those at greatest risk for Barrett’s oesophagus improve cost-effectiveness.58 Guidelines published by the British Society of Gastroenterology in 2014 recommend that endoscopic screening be considered in patients with chronic gastro-oesophageal reflux symptoms and multiple risk factors for Barrett’s oesophagus (at least three of aged 50 years or older, Caucasian background, male sex, and obesity). They suggest that the threshold of multiple risk factors should be lowered in the presence of a family history including at least one first-degree relative with Barrett’s oesophagus or oesophageal adenocarcinoma.59
For more widespread Barrett’s oesophagus screening to be considered, the costs of detection need to be reduced substantially with no compromise in accuracy. Studies of less costly screening methods (eg, ultrathin endoscopes, cytosponges) have yielded promising results but it is too early for these to be recommended at a population level.60,61
Endoscopic surveillance
Surveillance is the strategy of systematically following up patients with a known precursor condition to reduce (or prevent) the harms of cancer progression. The aim is to detect progression early, so that disease can be treated with the least invasive method, thereby reducing morbidity and mortality from cancer. The decision to commence endoscopic surveillance should be individualised for each patient, after considering factors such as age, comorbidities and the patient’s wishes and ability to participate in a long term surveillance program.
Endoscopic surveillance in Barrett’s oesophagus involves careful and meticulous examination of the Barrett’s segment with a high resolution white light endoscope, followed by biopsies from the segment. It is recommended that biopsies be taken according to the Seattle protocol, with biopsies of any mucosal irregularity (labelled separately) and quadrantic biopsies every 2 cm, unless there is known or suspected dysplasia, in which case quadrantic biopsies should be taken every centimetre. In the presence of erosive oesophagitis, it is recommended that acid suppression be maximised and surveillance endoscopy repeated in 2–3 months. This allows the oesophagitis to heal, thereby permitting underlying (masked) lesions to be identified and further biopsies to be taken.
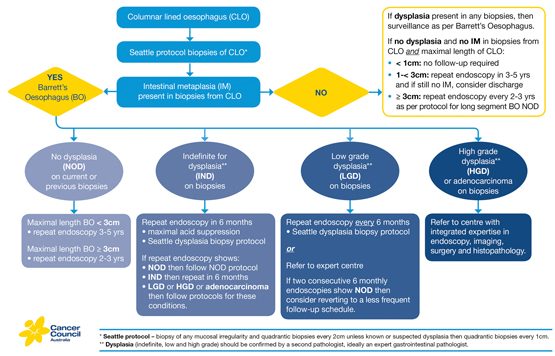
The interval between surveillance endoscopies depends on segment length and the presence of dysplasia (Box 3). In patients with Barrett’s oesophagus with no current or past dysplasia, follow-up endoscopy is recommended every 2–3 years in those with long segment disease, and every 3–5 years in those with short segment disease. Follow-up and management of patients with dysplasia is discussed below.
In some patients, a columnar-lined oesophagus is found at endoscopy but no intestinal metaplasia or dysplasia is seen histologically. The biological implications of this finding remain uncertain. The Australian guidelines recommend follow-up intervals based on segment length: < 1 cm, no endoscopic follow-up; 1–< 3 cm, 3–5 years; and ≥ 3 cm, 2–3 years.4
Although endoscopic surveillance in Barrett’s oesophagus is the current recommended practice, there is no direct evidence from randomised trials for its effectiveness or cost-effectiveness. Economic modelling studies suggest that current surveillance practices are unlikely to be cost-effective, and that identifying patients at high risk of progression to oesophageal adenocarcinoma substantially improves cost-effectiveness.39,62,63 The future hope is that a combination of clinical, endoscopic, blood or tissue markers might be used to develop risk stratification tools for identifying high risk patients most likely to benefit from surveillance and early intervention.64
Management of gastro-oesophageal reflux disease
In patients with Barrett’s oesophagus and gastro-oesophageal reflux symptoms, proton pump inhibitor treatment is recommended at a dose titrated to control symptoms and heal reflux oesophagitis. If proton pump inhibitors fail to control gastro-oesophageal reflux symptoms or heal reflux oesophagitis, surgical fundoplication can be considered.5 There is no strong evidence to suggest that medical or surgical therapy of gastro-oesophageal reflux disease leads to any substantial regression in segment length or influences progression to cancer.65
Management of low grade dysplasia
Management of LGD patients is currently uncertain, as new data suggest cancer progression rates are higher in patients whose LGD has been confirmed by an expert pathologist.48 A recent multicentre European randomised study of radiofrequency ablation in patients with expert-confirmed LGD found that control patients undergoing intensive endoscopic surveillance had a progression rate to cancer of 8.8%, while patients in the intervention arm had a progression rate to cancer of 1.5%.66 The Australian guidelines recommend that those with LGD be either closely monitored with frequent endoscopic assessment and biopsies every 6 months or referred to an expert centre for ongoing follow-up and consideration of ablative therapy of the Barrett’s segment.4 The decision regarding management of patients with LGD needs to take into account the features of the Barrett’s segment and histology as well as patient age, fitness and preference.
Management of indefinite for dysplasia
Indefinite for dysplasia is reported when biopsies from the Barrett’s segment show some histological features of true dysplasia but other processes (eg, inflammation) cannot be excluded as a cause for the changes. As with LGD and HGD, such biopsies should be reviewed by a second pathologist, ideally an expert gastrointestinal pathologist. If indefinite for dysplasia remains the diagnosis, then Australian guidelines recommend that the patient be placed on maximal acid suppression and undergo repeat endoscopy with dysplasia protocol biopsies in 6 months.4
Management of high grade dysplasia and early oesophageal adenocarcinoma
Patients with HGD or early oesophageal adenocarcinoma should be referred to an expert centre that has integrated expertise in endoscopy, imaging, surgery and histopathology. This allows the initial diagnosis to be confirmed by a second pathologist (ideally an expert gastrointestinal pathologist) and allows assessment and management by a multidisciplinary team.
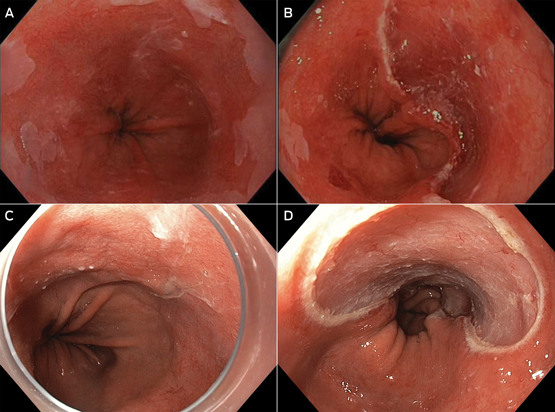
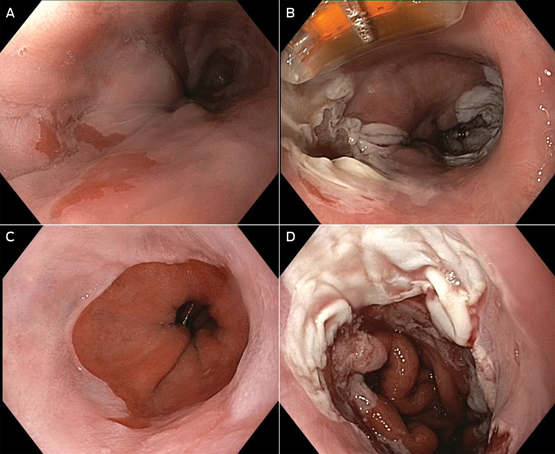
Until a decade ago, the only definitive management option for patients with HGD or early oesophageal adenocarcinoma was oesophagectomy. Because of the low risk of metastatic disease in cancers confined to the mucosa (1–2% for T1a lesions),67 the past decade has seen a number of endoscopic techniques developed to manage these conditions. These techniques can be divided in to two groups: resection (endoscopic mucosal resection [EMR] and endoscopic submucosal dissection); and ablation (radiofrequency ablation [RFA], argon plasma coagulation, photodynamic therapy and cryotherapy). In Australia, EMR and RFA are the most commonly used resection and ablation techniques, respectively (Box 4 and Box 5). In EMR, the oesophageal mucosa is aspirated into a cap on the end of the endoscope, a band applied and the captured mucosa and submucosa resected and retrieved endoscopically. In RFA, thermal injury delivered by an endoscopically placed device is used to destroy the oesophageal mucosa. Although these endoscopic methods do carry a small risk of complications (pain, bleeding, perforation and stricture formation), they are substantially less morbid, less expensive and more organ-preserving than surgery.68
Initial management of patients with histologically confirmed HGD and early oesophageal adenocarcinoma involves detailed endoscopic assessment and staging of the Barrett’s segment, with EMR of any visible lesions and biopsies of the Barrett’s segment according to the Seattle protocol. EMR of visible lesions enables accurate histological staging of the depth of invasion; studies have shown that EMR can change staging assessments in 48% of patients.69
Endoscopic treatment of high grade dysplasia
In patients with HGD without adenocarcinoma, further endoscopic treatment of the remaining Barrett’s segment is advised because of the risk of metachronous lesions. Treatment options for the residual flat segment vary from patient to patient, depending on factors such as segment length, the presence of a circumferential segment or the presence of an oesophageal stricture and involve EMR and/or endoscopic ablation. Follow-up studies of endoscopic therapy in HGD have shown promising long term results, with complete eradication of dysplasia and metaplasia in 89% of patients at 2 years.70 Longer term outcome studies are awaited. Post-treatment oesophagitis may be associated with decreased success rates of endoscopic therapy. It is therefore recommended that endoscopically treated patients receive ongoing medical therapy with a proton pump inhibitor to control gastro-oesophageal reflux symptoms and to prevent and heal oesophagitis.5 If medical therapy is unable to achieve these goals, surgical fundoplication may be considered. Long term, frequent endoscopic surveillance following treatment is recommended because of the risk of recurrence and metachronous lesions.
Endoscopic treatment of early oesophageal adenocarcinoma
In patients with early oesophageal adenocarcinoma and favourable histology (T1a; size, < 2 cm; well differentiated grade; no lymphovascular invasion; clear resection margins), further endoscopic treatment of the remaining Barrett’s segment can be planned.71 Treatment of the residual segment is advised because of the risk of future metachronous lesions within the segment. The endoscopic method for treating the residual flat dysplastic and non-dysplastic mucosa varies depending on patient factors, but will typically involve EMR and/or RFA. Australian guidelines recommend that ablation should only be used to treat flat dysplastic and non-dysplastic mucosa, and not as primary endoscopic therapy for early oesophageal adenocarcinoma.4
Because of the higher risks of lymph node metastases in T1b lesions (12–50%), surgically fit patients with T1b lesions should be offered oesophagectomy as a potentially curative treatment.40,72 In those patients who are unfit or unwilling to have surgery, endoscopic treatment with or without adjuvant therapy can be offered, but recognising the significant risk of lymph node metastasis that will remain undiminished by endoscopic therapy.73,74
Metachronous lesions or recurrent oesophageal adenocarcinoma have been described in up to 15% of patients undergoing endoscopic therapy for T1a lesions; therefore, long term, frequent post-treatment endoscopic surveillance is recommended. In most cases, lesions found on surveillance can be successfully managed endoscopically, with an overall 94% long term complete remission rate.75 In patients for whom endoscopic therapy is unsuccessful or not appropriate, oesophagectomy should be considered. Surgery in patients with HGD or early oesophageal adenocarcinoma carries a lower perioperative mortality rate (1.6%) than surgery for more advanced oesophageal adenocarcinoma.76
Conclusion
Barrett’s oesophagus describes a metaplastic change to the epithelium of the lower oesophagus that predisposes the person affected to oesophageal adenocarcinoma. While risks of progression are not as high as previously assumed, they are not insignificant, posing a challenge for clinical management. Australian guidelines have been developed to assist practitioners in this area.4 New endoscopic techniques for treating dysplasia and early adenocarcinoma are now available that have markedly lower morbidity than older approaches. In the future, it is possible that new screening and surveillance technologies may prove cost-effective for identifying and managing patients with Barrett’s oesophagus in the community.
Box 1 –
Biopsies from normal oesophagus and Barrett’s oesophagus
Box 2 –
Endoscopic classification of Barrett’s oesophagus using the Prague criteria7
Box 3 –
Algorithm for recommended endoscopic surveillance schedule for Barrett’s oesophagus
Box 4 –
Endoscopic mucosal resection (EMR)
Box 5 –
Radiofrequency ablation (RFA)

 more_vert
more_vert