With a mean of 43.8 new cases diagnosed per 100 000 individuals in 2008, Australia has one of the highest age-standardised colorectal cancer (CRC) incidence rates worldwide, accounting for 12.7% of total cancers and 10% of all cancer deaths nationally.1
Survival from CRC highly depends on stage at diagnosis, and clinical trials have demonstrated that screening using faecal occult blood testing (FOBT) increases the detection rate of early-stage disease and reduces CRC mortality.2 Further, the efficacy of endoscopic polypectomy in preventing adenomas from progressing to CRC has led to a decrease in the incidence of CRC in screening trials.3 As a result, population-based screening programs to reduce mortality from CRC have been implemented in many nations in recent years.4–6
In Australia, national guidelines for CRC screening were introduced in 1999, recommending asymptomatic persons aged 50 years and over be screened using FOBT at least every 2 years.7 However, CRC screening tests were not freely available until the National Bowel Cancer Screening Program (NBCSP) was launched in 2006 with a one-off faecal occult blood test mailed to all people turning 55 and 65, and, from 2008, additionally to people turning 50.4 Although NBCSP-detected cancers are being diagnosed at an earlier stage than symptomatic cancers,8,9 the effects of opportunistic screening that occurred before the NBCSP on CRC incidence remain largely unexplored. Observational data on CRC incidence after CRC screening do not directly indicate screening effectiveness in reducing incidence, as cancers diagnosed after screening constitute those either missed at screening or arising de novo during the interval between screens (interval cancer), and a reduced incidence compared with non-screened individuals is to be expected. However, such data are useful in providing a real-world indication of the likely outcomes and health services use after CRC screening in the general population and giving indirect insights into the impact of screening.10,11
In this study, we present data on the relationship between reported CRC screening history and CRC incidence in a large population-based Australian cohort study, the 45 and Up Study.
Methods
Study population
The 45 and Up Study is a population-based Australian cohort study designed to investigate healthy ageing.12 Briefly, eligible participants were randomly selected from the Australian universal health insurance records (Medicare Australia). A total of 267 113 individuals (123 906 men and 143 207 women) aged ≥ 45 years from the general population in New South Wales joined the study by completing a postal questionnaire (distributed from January 2006 to December 2008) and giving written consent. Ethics approval for the study was provided by the University of New South Wales Human Research Ethics Committee and the NSW Population and Health Services Research Ethics Committee.
We excluded participants with pre-existing cancer (other than non-melanoma skin cancer), missing date of study entry, body mass index (BMI) outside the range of 15–50 kg/m2, and with invalid or most likely implausible values for physical activity and diet as previously defined.13 We excluded 55 777 participants with pre-existing cancer (other than non-melanoma skin cancer), 11 with missing date of study entry, 2113 with BMI outside the range of 15–50 kg/m2 and 12 759 with invalid or most likely implausible values for physical activity and diet. Exclusions left 196 464 participants for analysis.
Exposure assessment and definitions of variables
Information on all variables was derived from the self-administered questionnaire.14 The questionnaire included information on sociodemographic characteristics, medical history, body weight and height, smoking, alcohol, diet and physical activity.
For screening history, participants were asked whether they had ever been screened for CRC, and if so which test they had undergone (FOBT, sigmoidoscopy or colonoscopy). Year of most recent test was also recorded. The following exposure groups were defined: ever versus never screened, time since last screening (never, screened ≤ 3 years ago, screened > 3 years ago), screening modality (ever FOBT v never screened; ever endoscopy v never screened) and time since last screening according to single screening procedures. Initially, no distinction was made between primary screening by endoscopy or endoscopy after another screening procedure, most likely FOBT. However, we separately evaluated primary screening by endoscopy in additional analyses.
Ascertainment of colorectal cancer
Information on cancer incidence was obtained through record linkage with the NSW Central Cancer Registry. For our analysis, the specific censoring date at which the cancer registry was considered complete was 31 December 2008. Registry information was complemented with record data from the NSW Admitted Patient Data Collection (APDC) for the period from 1 January 2009 to 31 December 2011. The APDC is a complete census of all hospital admissions and discharges in NSW and contains, among other details, the principal reason for admission. As the primary treatment of CRC is surgical, the APDC is considered to provide reliable independent data on such diagnoses. Recent studies on CRC and breast cancer have shown that cancer diagnosis can be accurately identified using hospital data.15,16
We only considered first primary incident cases of CRC and participants were followed up from study entry to cancer diagnosis, death or follow-up termination (31 December 2011), whichever came first. Incidence data were coded using the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3), with CRC comprising C18–C20 (excluding C18.1, cancers of the appendix). Proximal colon tumours included the caecum, ascending colon, hepatic flexure, and transverse colon (C18.0, 18.2–18.4). Distal colon tumours included the splenic flexure (C18.5), and descending (C18.6) and sigmoid (C18.7) colon. Overlapping lesions (C18.8) and unspecified colon (C18.9) were grouped among all colon cancers only (C18.0, C18.2–C18.9). Cancer of the rectum included tumours occurring at the rectosigmoid junction (C19) and rectum (C20). Anal canal tumours were excluded.
Statistical analysis
Associations between history of CRC screening and incidence of CRC were investigated by calculating hazard ratios (HRs) using proportional hazards regression stratified by age. Age was taken as the underlying time metric, with entry and exit time defined as the participant’s age at recruitment and age at cancer diagnosis or censoring, respectively. HRs are presented in relation to never screened individuals and are adjusted for sex; BMI (per kg/m2); highest qualification (no school, intermediate/high school, apprenticeship, university), household income (< $20 000, $20 000–$49 999, $50 000–$69 999, and ≥ $70 000); remoteness based on the Accessibility/Remoteness Index of Australia (city, regional, remote); a diagnosis of diabetes; family history of CRC; aspirin use; smoking (never, former, current); alcohol use (drinks/day); vigorous physical activity (none, 0 to 1 hour/week, > 1 to 3.5 hours/week, > 3.5 hours/week); and intake of red meat, processed meat, cereals, fruits, vegetables and wholemeal bread.
To appropriately handle missing data (up to 13% missing data for some variables), we used multiple imputation techniques (PROC MI and PROC MIANALYZE in SAS).17 All variables included in the multivariate model were included in the imputation procedure, and five imputation cycles were performed.
In sensitivity analyses, we excluded cases occurring during the first 2 years of follow-up and restricted the analysis to individuals with complete information on all variables to compare results with those obtained using multiple imputation.
All analyses were performed using SAS, version 9.3 (SAS Institute) and two-sided P values were considered.
Results
During a mean follow-up of 3.78 years (SD, 0.92 years) — 741 829 person-years — a total of 1096 cases of incident CRC accrued (454 proximal colon, 240 distal colon, 349 rectal and 53 unspecified cancers).
Screened individuals were more likely to have a family history of CRC and less likely to be current smokers than unscreened individuals (Box 1).
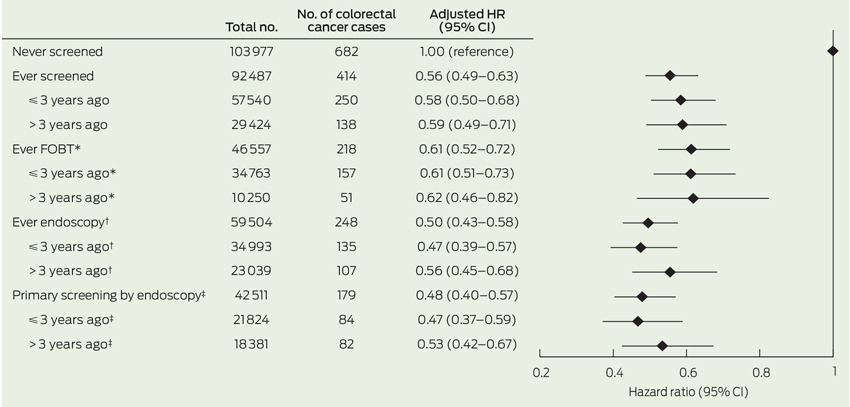
Having ever been screened before baseline was associated with a 44% reduced risk of developing CRC during follow-up in comparison to never having been screened (HR, 0.56; 95% CI, 0.49–0.63) (Box 2). HRs of CRC were 0.61 (95% CI, 0.52–0.72) and 0.50 (95% CI, 0.43–0.58), respectively, among those reporting ever having had FOBT and those reporting endoscopy versus no screening. We observed negligible differences in the HRs for those screened more than 3 years versus 3 years or less before baseline.
In relation to CRC subtypes, the inverse association between all screening exposure variables was strongest for rectal (HR, 0.35; 95% CI, 0.27–0.45) followed by distal cancer (HR, 0.60; 95% CI, 0.46–0.78), while the relationship was weaker for proximal colon cancers (HR, 0.76; 95% CI, 0.62–0.92) (Box 3).
Similar HRs were observed in subgroup analyses; for example, men and women and across smoking status (data not shown). The risk of developing CRC was particularly low for individuals with a family history of CRC (HR, 0.38; 95% CI, 0.27–0.54) compared with individuals without (HR, 0.59; 95% CI, 0.52–0.68) (P for interaction, 0.02).
Restricting the analysis to individuals with complete information on all variables (626 CRC cases) did not materially alter the results (data not shown). Exclusion of patients diagnosed during the first 2 years of follow-up slightly attenuated results for FOBT (HR, 0.72; 95% CI, 0.58–0.90), while estimates remained virtually unchanged for ever having had an endoscopy (HR, 0.52; 95% CI, 0.42–0.65).
Discussion
In this large population-based prospective study, a history of CRC screening was related to a 44% lower risk of subsequent CRC compared with never having undergone screening. Reductions in CRC risk were about 40% for ever having had FOBT and 50% for endoscopic procedures, compared with never having undergone CRC screening. The reduction in CRC incidence was observed up to 4 years after baseline and applied most strongly to rectal cancer.
Although our data cannot be used to directly evaluate CRC screening effectiveness, they are largely consistent with trial-based evidence on the efficacy of CRC screening in reducing CRC incidence and mortality. A meta-analysis of four randomised controlled trials (RCTs) indicated that those allocated to FOBT had a 16% reduction in CRC mortality compared with those not randomised to screening,18 while risk reduction may be even greater among those who adhere to a screening program.19 Based on data from five RCTs, sigmoidoscopy-based screening reduced CRC incidence by 18%.3 Colonoscopy is considered the “gold standard” for examination of the entire colon and rectum; however, its efficacy in relation to CRC incidence and mortality has never been investigated in RCTs and its magnitude of effect is currently unknown.20
Our observation of a 50% lower CRC risk after screening endoscopy is in line with two observational prospective studies from the United States.10,11 Interestingly, the reduction in CRC risk after endoscopy was greater for rectal and distal colon cancers than tumours located in the proximal colon. In our study, we reported combined estimates for sigmoidoscopy and colonoscopy. Because flexible sigmoidoscopy only allows inspection of (and polyp removal from) the distal but not proximal colon, our finding of stronger risk associations with distal CRC might be due to the lack of association of sigmoidoscopy with proximal colon cancer. However, only a small proportion of screened individuals reported having undergone sigmoidoscopy (< 6% v 64% colonoscopy and 52% FOBT), with 70% of them additionally having had colonoscopy, presumably as a follow-up of abnormal sigmoidoscopy. Our risk estimates are thus likely to be driven by the effect of colonoscopy. Differences in the strength of association with colonoscopy for proximal and distal colon cancers have been noted previously.11,21,22 A recent long-term prospective study from the US found reductions in incidence of 30% and 75% for proximal and distal CRC after colonoscopy, respectively, which compares well with our finding.11 Possible explanations for the difficulties in detecting precancerous lesions in the proximal colon may include incomplete colonoscopies or poor quality of bowel preparation resulting in missed lesions; flat lesions which are difficult to detect and remove; and the occurrence of rapidly growing cancers.21,23 Large, long-term RCTs on the effectiveness and the magnitude of effect of colonoscopy on the incidence of CRC and its subtypes in the general population are needed and underway, but results are unlikely to be available within the next 10 years.24,25
The association of FOBT with reduced CRC incidence appeared to be driven primarily by the strong relation of FOBT with rectal cancers in our study. The ability of FOBT to detect proximal colon cancers has been debated.26 FOBT exploits the tendency of CRC and large adenomas to bleed; however, haemoglobin from proximal neoplasia may degrade on passage to the anus, which may affect the accuracy of the test. In a systematic review of seven prospective screening studies, most of the studies indicated lower sensitivity for detecting proximal advanced neoplasia than for distal advanced neoplasia.26 In general, sensitivity for detecting precancerous lesions is low for guaiac faecal occult blood tests (16%–31%) and moderate for the newer faecal immunochemical tests (27%–67%).27
The lack of differences in the association of screening with CRC incidence across the two periods may be due to the relatively short time frame of our study. Case–control studies have suggested that the effect of endoscopy sustains for more than 10 years with little attenuation.28,29 For FOBT, the effect attenuation we observed after excluding cases occurring during the first 2 years of follow-up may reflect its ability to detect early-stage disease and its rather low to moderate sensitivity for detecting precancerous lesions.27
The results of our study need to be interpreted against the backdrop of its limitations. First, as has been noted, the results cannot be directly translated into effectiveness of population screening. Greater emphasis should be placed on the data as providing insights into the likely experiences of consumers and on health services use after CRC screening. Second, assignment of screening status was based on self-report and may be subject to misreport. A recent meta-analysis, however, indicates self-reports of CRC screening to be reasonably reliable.30 Although the questionnaire explicitly asked for screening, we cannot rule out that some participants may have reported having had endoscopy as a diagnostic test (due to symptoms) rather than as true screening (as part of a routine health check-up), and our risk estimates may not entirely represent the effect of true screening. This potential mixture of effects, however, will most likely have biased our estimates towards the null, and the protective effect of true screening by endoscopy might be even stronger. Third, we were not able to take into account information about the number of previous screens and a potential diagnosis or removal of polyps. Removal of polyps would have altered individuals’ CRC risk. Further, people having polyps removed would be more likely to participate in screening regularly. People screened more than once will have a lower risk of CRC irrespective of whether screening is beneficial, as they must not have had CRC detected on a previous screen and will later be diagnosed only if the last screen produced a false-negative result or cancer developed afterwards. Similarly, because we excluded pre-existing CRC at baseline, all incident cases must have had either a false-negative screen or developed cancer since last screening. Fourth, we lacked information on the type of FOBT and sigmoidoscopy. Finally, we had no information on screening during follow-up; however, the likelihood of bias due to repeated screening appears low, given the relatively short follow-up in our study.
In conclusion, this population-based prospective study illustrates a lower CRC risk among individuals with a history of CRC screening, compared with individuals who have never had CRC screening, through either FOBT or endoscopy, lasting for at least 4 years after screening.
1 General characteristics of the 196 464 included participants from the 45 and Up Study, by screening history
| |
Never screened (n = 103 977)
|
Ever screened (n = 92 487)
|
P for comparison
|
|
|
Age, mean (SD)
|
59.7 (11.1)
|
62.8 (10.1)
|
< 0.001
|
|
Body mass index, mean (SD)
|
27.0 (5.0)
|
26.9 (4.8)
|
< 0.001
|
|
Men
|
41.1%
|
44.6%
|
< 0.001
|
|
Diabetes
|
8.0%
|
8.1%
|
0.95
|
|
University education
|
23.8%
|
25.3%
|
< 0.001
|
|
Yearly household income ≥ $70 000
|
27.1%
|
26.8%
|
0.06
|
|
Remote
|
2.3%
|
1.8%
|
< 0.001
|
|
First-degree relative with CRC
|
7.2%
|
20.0%
|
< 0.001
|
|
Regular aspirin use
|
17.1%
|
22.5%
|
< 0.001
|
|
Former smokers
|
32.7%
|
36.0%
|
< 0.001
|
|
Current smokers
|
9.7%
|
5.4%
|
< 0.001
|
|
Alcoholic drinks per day*
|
1.47
|
1.50
|
< 0.001
|
|
Vigorous activity > 3.5 hours/week
|
8.8%
|
8.6%
|
0.18
|
|
Red meat ≥ 5 times/week
|
20.9%
|
22.4%
|
< 0.001
|
|
Processed meat ≥ 5 times/week
|
5.4%
|
5.0%
|
< 0.001
|
|
Fruits ≥ 2 serves/day
|
55.9%
|
60.7%
|
< 0.001
|
|
Vegetables ≥ 5 serves/day
|
32.8%
|
36.0%
|
< 0.001
|
|
Breakfast cereals > 1 times/week
|
76.5%
|
82.9%
|
< 0.001
|
|
Wholegrain bread ≥ 5 serves/week
|
47.1%
|
51.6%
|
< 0.001
|
|
|
CRC = colorectal cancer. * Among drinkers only.
|
2 Adjusted hazard ratios for the associations between different screening procedures and incidence of colorectal cancer in the 45 and Up Study
3 Adjusted hazard ratios for the associations between different colorectal cancer (CRC) screening procedures to the incidence of different subtypes of CRC in the 45 and Up Study (n = 196 464)
| |
|
Proximal colon (n = 454)
|
Distal colon (n = 240)
|
Rectum (n = 349)
|
|
Screening variables
|
Total no.
|
No.
|
Adjusted HR (95% CI)
|
No.
|
Adjusted HR (95% CI)
|
No.
|
Adjusted HR (95% CI)
|
|
|
Never screened (reference)
|
130 977
|
245
|
1.00 (reference)
|
149
|
1.00 (reference)
|
253
|
1.00 (reference)
|
|
Ever screened
|
92 487
|
209
|
0.76 (0.62–0.92)
|
91
|
0.60 (0.46–0.78)
|
96
|
0.35 (0.27–0.45)
|
|
≤ 3 years ago
|
57 540
|
128
|
0.82 (0.66–1.03)
|
56
|
0.63 (0.46–0.87)
|
57
|
0.33 (0.25–0.45)
|
|
> 3 years ago
|
29 424
|
68
|
0.76 (0.58–1.00)
|
30
|
0.62 (0.42–0.92)
|
34
|
0.38 (0.27–0.54)
|
|
Ever FOBT*
|
56 557
|
106
|
0.87 (0.69–1.10)
|
53
|
0.72 (0.52–1.00)
|
52
|
0.36 (0.26–0.49)
|
|
≤ 3 years ago*
|
34 763
|
76
|
0.83 (0.64–1.09)
|
36
|
0.69 (0.47–1.01)
|
39
|
0.36 (0.25–0.51)
|
|
> 3 years ago*
|
10 250
|
24
|
0.79 (0.52–1.21)
|
15
|
0.88 (0.51–1.50)
|
12
|
0.35 (0.20–0.64)
|
|
Ever endoscopy†
|
59 504
|
133
|
0.73 (0.58–0.90)
|
46
|
0.46 (0.33–0.65)
|
58
|
0.33 (0.25–0.45)
|
|
≤ 3 years ago†
|
34 993
|
73
|
0.68 (0.52–0.89)
|
26
|
0.47 (0.30–0.72)
|
31
|
0.30 (0.20–0.43)
|
|
> 3 years ago†
|
23 039
|
56
|
0.74 (0.55–1.00)
|
20
|
0.52 (0.32–0.84)
|
26
|
0.38 (0.25–0.57)
|
|
Primary screening by endoscopy‡
|
42 511
|
92
|
0.71 (0.55–0.90)
|
30
|
0.43 (0.29–0.64)
|
38
|
0.32 (0.23–0.45)
|
|
≤ 3 years ago‡
|
21 824
|
49
|
0.72 (0.52–0.98)
|
16
|
0.45 (0.27–0.76)
|
16
|
0.26 (0.16–0.43)
|
|
> 3 years ago‡
|
18 381
|
42
|
0.70 (0.50–0.97)
|
14
|
0.45 (0.26–0.79)
|
21
|
0.39 (0.25–0.61)
|
|
|
HR = hazard ratio. FOBT = faecal occult blood testing. HRs were obtained from proportional hazards regression, and were stratified by age and adjusted for demographic and health-related variables as described in the text, in addition to footnoted adjustments.* Adjusted for having undergone primary endoscopy. † Adjusted for having undergone FOBT only. ‡ Adjusted for having undergone FOBT, or FOBT and endoscopy.
|

 more_vert
more_vert