Physical inactivity might lead to excess cardiovascular and cancer mortality, whereas compliance with the WHO-recommended moderate and vigorous physical activity (MPVA) of 150 min per week as part of a healthy lifestyle could reduce that risk. One could argue that this is largely a matter of public education, but if people can be convinced, do they have sufficient opportunities to act accordingly? James Sallis and colleagues1 (May 28, p 2207) showed that the design of the urban environment has an important effect on physical activity levels of residents.
Preference: Education
190
GPs win an ePIP breather
Medical practices being pushed to the financial brink by the Medicare rebate freeze and other Government cuts have won a partial reprieve after Health Minister Sussan Ley pushed back the deadline on shared health summary uploads to early next year.
In a breakthrough following intense lobbying by the AMA, Ms Ley has advised GPs will be given until 31 January 2017 to comply with new rules that require practices to upload shared health summaries (SHS) for at least 0.5 per cent of patients every quarter to remain eligible for the Practice Incentive Program Digital Health Incentive.
AMA President Dr Michael Gannon, who has raised the issue at a several meetings with the Minister, said the decision was “very welcome”.
“GPs are already under significant financial pressure from the Medicare rebate freeze and other funding cuts, and the last thing they needed was to also lose vital PIP incentive payments,” he said.
The Government originally required practices to comply with the new eligibility criteria from May this year, but the AMA warned at the time that this would be unworkable for many practices and risked undermining the goodwill of GPs which was essential to making the My Health Record system a success.
In June, the AMA called for a moratorium on the new rules after a survey it conducted found that just 24 per cent of practices considered themselves able to comply, while almost 40 per cent said they would not be able to and 36 per cent were unsure.
Government figures show that in the first three months of operation, 1500 practices failed to meet their SHS upload target and 69 practices withdrew from the scheme altogether.
Dr Gannon said failure to comply had the potential to deliver a heavy financial blow to practices already under substantial financial pressure.
“If the Government had not relaxed its approach, close to a third of previously eligible general practices faced losing significant financial support,” the AMA President said. “In many cases, practices would have been more than $20,000 worse off. With so many already close to breaking point, this could have been disastrous.”
The Minister’s decision follows a resolution passed by the AMA Federal Council in August calling for a moratorium on the new upload requirements and urging the Government to investigate the reasons why so many practices were struggling to comply.
The Federal Council said the Government should get the Practice Incentive Program Advisory Group (PIPAG) to conduct the review and provide recommendations on what could be done to improve practice compliance.
Dr Gannon said the episode highlighted the importance of the Government heeding the views and advice of general practitioners and their representatives.
The Government had pushed ahead with its SHS requirements against the advice of all the GP groups sitting on PIPAG, and the AMA President said in future it should ensure that any changes to the PIP Digital Health Incentive were based on the Advisory Group’s advice.
Dr Gannon said the medical profession strongly supported the Government’s My Health Record, and the Minister’s decision to extend the SHS requirement deadline would help shore up the goodwill of GPs to support its successful implementation.
“It is pleasing that the Minister has recognised the concerns that have been consistently raised by the profession, and this decision provides some breathing space for practices,” Dr Gannon said.
“With adequate time, education, and support, many of the affected 1500 general practices may well begin to genuinely engage with the My Health Record, and eventually champion it.
“But it is important that the Government continues to review the implementation of the PIP Digital Health Incentive in consultation with PIPAG.
“We need to know why practices failed to comply, and ensure that any of these issues are addressed before the end of January deadline. If a large number of practices still cannot comply by the new deadline, we may still need to revisit the policy.”
Adrian Rollins
Primary amoebic meningoencephalitis in North Queensland: the paediatric experience
Primary amoebic meningoencephalitis (PAM) is a rare but fulminant disease leading to diffuse haemorrhagic necrotising meningoencephalitis, and has a very poor prognosis.1 Naegleria fowleri is the causative agent. At Townsville Hospital, our first confirmed case of PAM was an 18-month-old girl from a rural location in North Queensland who presented with fever, seizures and an altered level of consciousness.2 Organisms resembling Naegleria spp. were seen on microscopy of cerebrospinal fluid (CSF). Despite aggressive therapy with multiple antimicrobial agents, the patient died within 72 hours of presentation. An older sibling of the patient had presented with a similar syndrome several years earlier and had died of an undifferentiated meningitic illness. The sibling was retrospectively suspected to also have had PAM.2
Our second confirmed patient presented in early 2015. A previously well 12-month-old boy from a nearby West Queensland cattle-farming area had had a 36-hour history of fevers, rhinorrhoea and frequent emesis, which progressed to lethargy and irritability. Before arrival at the local rural hospital, he had a tonic–clonic seizure lasting 3–5 minutes. On arrival he appeared drowsy, had mottled skin, a blanching maculopapular rash, which may not necessarily have been related to PAM, and a central capillary refill of 3–4 seconds. He was treated with intravenous antibiotics for presumed bacterial meningitis. Given the remote location and clinical suspicion of elevated intracranial pressure, lumbar puncture was not performed. On arrival at Townsville Hospital, his Glasgow Coma Scale score was 8/15, he was increasingly febrile, and had an evolving maculopapular rash. Broad spectrum antimicrobial therapy was subsequently started for presumed meningoencephalitis. Within 18 hours of leaving home, he had no spontaneous respiratory effort, reduced tone, up-going plantar reflexes and fixed pupils.
Neuroimaging showed diffuse cerebral oedema with progressive dilation of the ventricular system on sequential studies. An external ventricular drain was placed because of clinical instability, and CSF microscopy showed motile trophozoites on a wet preparation and Giemsa stain, consistent with N. fowleri. The patient was commenced on intrathecal amphotericin, with no improvement in his clinical state. The organism seen in the CSF was confirmed after the patient’s death by polymerase chain reaction (PCR) analysis as being N. fowleri. When reviewing the patient’s history, it was noted that, as in previous cases, he lived on a property that used untreated and unfiltered bore water domestically, to which he had multiple potential exposures, including via water play with hoses and bathing.
Literature review
We searched the PubMed database using the terms “Naegleri”, “fowleri” and “meningitis”. No time period was specified. The James Cook University eJournal database was searched for historical information.
We also searched the Queensland Health Communicable Diseases Branch and the Communicable Diseases Network Australia databases for Australian cases, but, as N. fowleri infection is not a notifiable disease, this returned a low yield.
History of Naegleria fowleri
In 1899, the Austrian scientist Franz Schardinger published the first description of an amoeba that transforms into a flagellate, with drawings of the amoeba, cysts and flagellates. In 1912, Alexeieff coined the name Naegleria, but physicians at the time thought that the genus did not cause disease in humans.3 It was not until the late 1960s that Naegleria was implicated as the cause of PAM by the work of Adelaide pathologists Malcolm Fowler and Rodney Carter, and of South Australian rural general practitioner Robert Cooter. In 1965, it was first proposed that the organism entered the CSF through the cribriform plate after Fowler isolated the organism in autopsy specimens. Following communication of his findings, Cooter and colleagues were able to directly observe the live amoeba in a CSF sample from a 10-year-old boy who presented with meningoencephalitis.4,5
Pathophysiology
N. fowleri lives and multiplies in warm freshwater areas, and acquisition is often associated with water-based recreational activities.6 Infection may occur when contaminated water is flushed into the nasal cavity. After penetrating the nasal mucosa and passing through the cribriform plate, trophozoites migrate along the olfactory nerve directly into brain tissue. Cases are almost universally fatal, although survival has been reported in the literature following early diagnosis and management.7,8
Epidemiology
The worldwide incidence of PAM is not accurately known,9 and the disease is likely to be under-diagnosed and under-reported. In the developing world, numerous factors affect accurate identification, including a lack of resources or expertise in microbiological diagnosis; prioritising management of other infections that are more common; and cultural beliefs that prevent autopsies.9 Higher water temperatures, inadequate sanitation, unsafe water sources, and religious ablution practices, such as the use of Neti pots for nasal cleansing, could potentially increase the risk for acquiring PAM.10,11 N. fowleri is a thermophilic organism and would therefore be expected to occur more frequently in tropical areas; however, the majority of cases are reported from subtropical or temperate regions.12 In a study in Karachi, Pakistan, N. fowleri was recovered from 8% of 52 domestic water taps that were sampled.13
An epidemiological review of PAM cases in the United States showed that N. fowleri infections are rare and primarily affect younger males exposed to warm recreational freshwater in the southern states.14–16 There are two case reports of patients who acquired N. fowleri from using treated municipal water for nasal irrigation,17 and another patient who contracted the disease from inadequately treated municipal water.18
In Australia, Dorsch and colleagues reported 20 cases of PAM, 13 of which occurred between 1955 and 1972 in South Australia. These cases were attributed to household water that was piped overland for long distances,19 allowing it to be heated to temperatures that promoted growth of the amoeba.5 After the introduction of continuous water chlorination in 1972, only one further case was reported in South Australia in 1981.19 In Queensland, only three previous patients have been described in the literature: one from Mount Morgan who survived, one from Charters Towers,19 and one referred from North West Queensland to Townsville Hospital.2
Clinical challenges
Patients with PAM present with the same symptoms as those with bacterial meningitis, and clinical differentiation between the two conditions is impossible. Patients often have a history of recent exposure to warm fresh water, although the definite exposure event is not always identified.9 The incubation period ranges from 2 to 15 days, and presenting symptoms may include meningism, fever, confusion and signs of elevated CSF pressure, such as seizures or coma.14
Diagnosis is made more difficult in North Queensland by the vast distances between remote towns in the western part of the state. Townsville Hospital services an area of nearly 150 000 km2 and has the only dedicated paediatric intensive care unit north of Brisbane. Patients with PAM inevitably require intensive care unit management and tertiary level investigations. Obtaining CSF samples for formal microscopic diagnosis is often impossible in small clinics with limited medical imaging or local laboratory services, and where performing a lumbar puncture is contraindicated by symptoms of raised intracranial pressure. Because of the rarity of the infection, greater awareness of PAM among primary health care professionals is required in order to increase suspicion in a clinically compatible case. Most importantly, education about prevention is essential for the continued health of rural communities, of which local medical professionals are a vital part. To this end, recent guidelines for the management of encephalitis20 include assessing risk factors for this condition and performing appropriate testing, as described below.
Diagnostic challenges
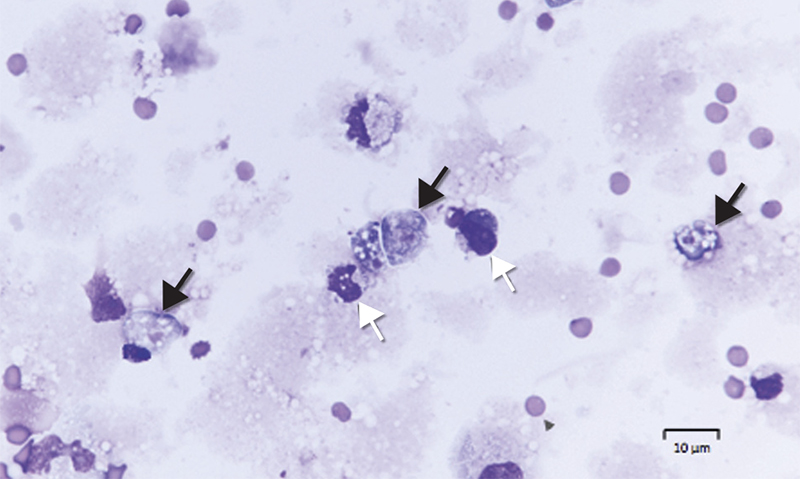
Diagnosis requires identification of motile trophozoites in CSF or characteristic morphology in stained specimens by a trained microbiologist (Box 1), with confirmation using molecular methods (PCR) or culture (Escherichia coli lawn culture). The trophozoites are visible in a wet unstained preparation of CSF (magnification, × 400), exhibiting sinusoidal movement by means of lobopodia; however, specimens need to be examined very soon after collection, as the amoebae degenerate rapidly in vitro and can be easily mistaken for leucocytes.
CSF chemistry is not diagnostic and will usually reveal a similar pattern to that of bacterial meningitis (Box 2). PCR analysis is performed using in-house methods at reference laboratories, and confirmation is often posthumous due to the rapid decline experienced by most patients. The US Centers for Disease Control and Prevention has developed a multiplex real-time TaqMan PCR assay to simultaneously identify three free-living amoebae (N. fowleri, Acanthamoeba spp. and Balamuthia mandrillaris) in clinical specimens.21 In Queensland, the pathology laboratory which performs all N. fowleri molecular testing uses primers and probes in line with the method of Qvarnstrom and colleagues.21 Culture may take several weeks and is difficult to perform.
Treatment
Given the limited data available, there are no set guidelines for antimicrobial therapy; however, it can be extrapolated from cases of patients who have survived that combination therapy with multiple anti-parasitic agents is required.
In 1969, Carter was able to demonstrate the sensitivity of the organism to amphotericin B (AMB) and it has remained the mainstay for treatment of PAM to this day.22 AMB has been used in all patients who have survived the illness.23 N. fowleri is highly sensitive to AMB in vitro with a minimum amoebicidal concentration of 0.01 μg/mL,24 and no resistance has been reported. Conventional AMB is preferred to liposomal forms as it can be given intrathecally as well as intravenously. Despite this, only a few patients have survived.25
Other antifungal drugs, such as miltefosine and the azoles, have all shown in vitro activity against N. fowleri.22–24 Miconazole has synergistic activity when combined with AMB, and fluconazole is used as first line in combination therapy.
Miltefosine is a protein kinase B inhibitor that was originally developed as an antineoplastic agent. It also has anti-parasitic activity and is used for the treatment of leishmaniasis. Schuster and colleagues26 reported that miltefosine showed in vitro activity against free-living amoebae, including N. fowleri, Acanthamoeba spp. and B. mandrillaris. Recently, miltefosine has been used in the treatment of Acanthamoeba granulomatous amoebic encephalitis and PAM. Linam and colleagues27 described the case of a child treated for PAM with combination therapy including amphotericin, miltefosine, fluconazole and rifampicin, who survived with no significant neurological sequelae.
Rifampicin is commonly used in the treatment of PAM; however, it has variable central nervous system penetration and poor efficacy in vitro.24 It may also reduce the efficacy of the azole drugs due to cytochrome P450 interactions. Although azithromycin has shown some in vitro and in vivo activity against N. fowleri, the other macrolides are less effective.9 Atypical agents such as the diamidines and chlorpromazine have been studied in animal models but have yet to be utilised clinically.24,28
Public health
As described, our patient was probably the third child to die with PAM in 14 years in a small area with a tiny population on remote Queensland cattle stations. As a response to the third death, a public health investigation found large numbers of N. fowleri at the patient’s homestead. In this district, water was sourced from deep artesian bores at about 60°C (Box 3) and cooled in open surface dams before being piped hundreds of metres on the surface to households, keeping water temperatures high. It was noted that the cases described in North Queensland were of children too young to be swimming in surface waters, the assumption being that they contracted the disease in the home environment. There had never been water treatment or filtration in the homesteads for generations; the clarity and taste of the bore water had often been a source of pride for owners. The difference in the present era of rural life was the advent of modern facilities, allowing the heated bore water to be pressurised via taps, hoses, toys and showerheads and delivered directly into the homestead.
The public health hypothesis was that:
-
Hot artesian bore water and long surface pipelines promote large concentrations of N. fowleri, which can be sucked into water pipes from sediments, particularly in drought years.
-
There had been no form of treatment for apparently clean water.
-
In recent years, among young families with modern water facilities, there were many more opportunities for water to be forced into a vulnerable (non-immune) child’s nose at pressure.
-
Simple filtration and disinfection of all water for washing and playing would prevent child deaths on these properties.
The public health dilemma was whether health promotion for a single, rare disease could be cost-effective or gain traction among rural people possibly reluctant to accept an expensive treatment of their water. Untreated surface water can also lead to a whole spectrum of gastrointestinal diseases, even if these were not familiar to the remote communities. It was decided that a health promotion campaign about domestic water filtration and treatment could protect not only from PAM but also from a range of other diseases.
The family of our second confirmed patient embarked on a rural education campaign of their own to prevent any further deaths from PAM or other waterborne diseases, culminating in an episode of the television series Australian Story in November 2015.29 To coincide with this story, public health physicians gave a series of talks to communities and health staff across a wide area of outback Queensland. To follow up the face-to-face campaign, Queensland Health released a safe water booklet with advice on cost-effective filtration and disinfection.30 As a result, many rural properties and some small towns are installing water treatment equipment for the first time. The South Australian and Western Australian governments have online education resources specifically targeting rural communities at risk of amoeba acquisition,31,32 with the primary focus on prevention. The aim of the Queensland public health booklet was to provide a more comprehensive education document for water treatment in rural communities.30
Conclusion
We hope an increased awareness of N. fowleri and its association with warm, non-chlorinated water provides an opportunity for counselling families about safe water use: avoiding diving or jumping into or squirting untreated water, and disinfecting or filtering water used for washing and playing, as well as for drinking. In particular, bore water at warm or hot temperatures and other warm water sources should be considered ideal reservoirs for this organism. In the clinical setting, difficulties with analysing CSF make it unlikely that an accurate diagnosis could be provided in a remote environment. The presentation of an acutely unwell child with a history of bore water exposure and signs of meningitis or encephalitis should, however, prompt consideration of PAM as a potentially life-threatening diagnosis. Our experience with this disease clearly demonstrates the crucial role of medical professionals working in rural and remote Australia in primary prevention of this almost universally fatal condition.
Box 1 –
Microscopy of cerebrospinal fluid of Patient 2,showing trophozoites (Giemsa stain, black arrows) and mononuclear leucocytes (white arrows)
Box 2 –
Analysis of cerebrospinal fluid (CSF) in patients with primary amoebic meningoencephalitis at Townsville Hospital
|
|
Microscopy |
White cell count (106/L) |
Polymorphonuclear leucocytes |
Protein (mg/L) |
CSF:blood glucose |
||||||||||
|
|
|||||||||||||||
|
Normal |
No organisms |
< 1 |
0 |
< 0.4 |
> 0.6 |
||||||||||
|
Patient 1 |
Motile trophozoites |
7200 |
91% |
3900 |
0.17 |
||||||||||
|
Patient 2 |
Motile trophozoites |
240 |
54% |
2700 |
0.12 |
||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 3 –
Great Artesian Basin
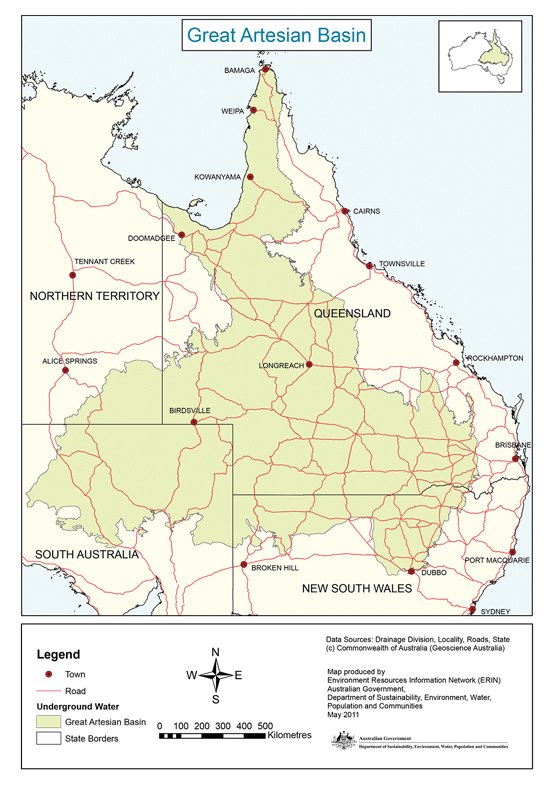
The Great Artesian Basin, from which bore water comes, covers a vast area of rural Australia. Western Queensland has a particularly wide coverage, and rural properties use bore water extensively.
Source: Australian Government Department of Sustainability, Environment, Water, Population and Communities, 2011. Available at http://www.agriculture.gov.au/water/national/great-artesian-basin (accessed Aug 2016).
Recruit local to relieve rural doctor shortage
The AMA has intensified its calls for the Federal Government to boost its investment in rural GP education amid mounting evidence that doctors who grow up and train in the bush are far more likely to practice there.
A study published in the latest edition of the Medical Journal of Australia found there is up to a 90 per cent chance that doctors who have a rural background and train in a rural area will still be practising in the bush five years later.
The result lends weight to AMA proposals for increased training opportunities for aspiring GPs and other specialists interested in practising in the country.
AMA President Dr Michael Gannon said the findings showed that the right investments by Government could make a real difference to access to care for rural communities.
“This study provides some important lessons for policy makers looking at how we can ensure that Australians living in rural areas have access to medical care,” Dr Gannon said.
While there has been an explosion in the number of medical school graduates in the past decade, relatively few are opting to train and practice in the bush, which remains chronically under-served.
Governments continue to recruit doctors from overseas to help fill the gap – the Herald Sun has revealed they sponsored 2268 health professionals to enter the country on 457 visas last year, including 1692 GPs and registered medical officers, 228 registered nurses, 35 specialists, 38 psychiatrists,28 surgeons and 19 anaesthetists.
Dr Gannon said proposals to build more medical schools were misguided.
“The problem isn’t a shortage of medical graduates. With medical school intakes now at record levels, we don’t need more medical students or any new medical schools.
“What we need are more and better opportunities for doctors, particularly those who come from the bush, to live and train in rural areas. The evidence shows that they are the most likely to stay on and serve their rural community once that qualify.”
The MJA study, Vocational training of general practitioners in rural locations is critical for the Australian rural medical workforce, found “a strong association between rural training pathways and subsequent rural practice”.
“[The] findings suggest that the periods leading up to and immediately following the vocational training are critically important windows of opportunity for ensuring that appropriate policies optimise recruitment of GPs for rural practice and their subsequent retention,” the study’s authors said.
Dr Gannon said these conclusions backed a number of policy proposals developed by the AMA to boost access to care in rural areas, including:
• for the targeted intake of medical students from rural areas to be increased from a quarter to a third of all new enrolments;
• the establishment of a Community Residency Program to give prevocational doctors, particularly those in rural areas, with access to three-month general practice placements;
• an increase in the GP training program intake to 1700 places by 2018;
• an expansion of the Specialist Training Program to 1400 places by 2018, with priority given to rural settings, under-supplied specialties and generalist roles; and
• access to regional training networks to support doctors to train and remain in rural areas.
“The Federal Government has a wonderful opportunity to make a real and lasting difference by adopting these sensible, effective, evidence-based measures,” Dr Gannon said.
The Government has promised to appoint a Rural Health Commissioner to champion rural health issues, including developing a National Rural Generalist Pathway to help address the shortage of rural medical practitioners.
Adrian Rollins
Teaching approaches in medicine made easier

Teaching professional attitudes and basic clinical skills to medical students: a practical guide. Jochanan Benbassat. Springer, 2015 (143 pp, €51.99). ISBN 9783319200880.
Training, learning styles, role modelling and behaviours vary among doctors. As a result, students experience different approaches to teaching, which later shape their own approaches to patient interviewing, data collection and problem solving as doctors. Some doctors encourage patients’ narratives by using open-ended questions; others favour closed questions. This can leave medical students confused by the different techniques.
Teaching professional attitudes and basic clinical skills to medical students: a practical guide aims to help tutors, clinicians and teachers by providing an approach to teaching patient interviews, physical examination and clinical reasoning and by bringing the reader closer to the behavioural and social sciences.
The book is easy to read and provides a thorough description of the paradigm shift seen in the teaching of medicine in recent decades. It includes analyses of the difficulties in teaching patient interview and communication techniques, physical examination, data recording and clinical reasoning, and gives insightful and well researched pedagogical suggestions for different approaches to teaching aimed at improving students’ learning. The tables included help readers to quickly summarise the differences in teaching approaches, suggested priorities and important learning outcomes.
The book was written by Jochanan Benbassat, Professor of Medicine and Chair of Sociology of Health at the Ben-Gurion University of the Negev, Israel, from 1992 to 1997 and currently a research associate at the Myers-JDC-Brookdale Institute in Jerusalem.
Eliciting and responding to patient histories of abuse and trauma: challenges for medical education
Toward trauma-informed medical education
Traumatic experiences such as childhood abuse, family violence, elder abuse and combat exposure influence both physical and mental health, health-related behaviour, and the ways in which patients interact with medical practitioners.1,2 Despite greater knowledge of the pervasive sequelae of psychological trauma, the implications for medical practice and for medical education are not well articulated. Many doctors lack confidence and remain ill-informed or avoidant when dealing with patients’ psychological trauma.3,4 The consequences of this include non-recognition of somatisation and of psychiatric disorders, delay in instituting proper treatment, and costs to the patient and health care system of unnecessary investigations and treatments.5,6 Here, we discuss why and how we should better train doctors to elicit and respond to patient histories of trauma.
High prevalence of trauma and its clinical sequelae
The lifetime prevalence of exposure to traumatic events is high (74.9% in Australian adults).7 Most people who experience trauma do not develop mental illness; however, trauma and abuse are substantial contributors to the burden of mental and physical ill health. The risk of post-traumatic stress disorder after trauma is about 10%,8 but childhood abuse and neglect in combination with later life stress contribute to the development of mental illnesses as diverse as psychoses, depression, eating disorders and addictions, as well as a range of physical illnesses.2,9 There are also clear associations between past trauma and abnormal illness behaviour10 and, related to this, increased health care use.6 These sequelae of patient trauma pervade all medical specialties and also dentistry.1
Incorporating teaching about trauma in medical curricula: key issues
Although there are several studies that describe training interventions for specific forms of trauma,3,11,12 there is little in the literature on current practices in medical education, either in Australia or elsewhere. The diversity in general structure, content and methods of medical curricula as outlined by the Australian Medical Council (AMC) probably extends to trauma-relevant components.13 Despite this diversity, it is possible to offer initial considerations for trauma-informed education. We focus on six interrelated aspects: communication skills; knowledge of the health effects of trauma and abuse; knowledge about the effects of trauma and abuse disclosures on doctors and other health professionals; specific knowledge relevant to different medical specialities and settings; teaching formats and methods; and the need for a staged, incremental, integrated program, structured to achieve continuity between undergraduate, prevocational and specialist phases.
Communication skills curricula13 in pre-clinical and clinical phases afford opportunities for trauma-specific education, but are also relevant to junior hospital and specialty training. Common issues that need to be addressed include the personal discomfort many doctors experience asking about trauma and abuse; when not to screen for or discuss trauma; and when to seek advice from senior colleagues, as overconfidence can be harmful, leading to patient distress and even re-traumatisation. Communication skills education should extend to discussion of services relevant to different forms of trauma, such as social work, refuges, police and the courts. Here, it would be valuable for medical students to visit these settings or meet with workers from them.
Training needs to be realistic in that doctors often work in settings that are not conducive to asking about trauma, such as busy emergency departments, hospital wards that lack privacy, and overloaded outpatient clinics, often with a lack of psychiatric support. However, these realities should not be a pretext for avoiding clinically competent trauma-informed practice. We do not propose that doctors become trauma therapists; rather that they become competent in empathically recognising and eliciting information about trauma, and at effective referral of patients to relevant services, including psychology and psychiatry.
Exposure to patients who have experienced trauma or abuse evokes a range of psychological reactions in students and trainees, from normal discomfort and distress through to vicarious traumatisation.3,4,14 In addition, there may be unhelpful, if not harmful, responses: doctors may adopt an avoidant “don’t ask, don’t tell” style; over-investigate; refer patients to other clinicians; exhibit stigmatising attitudes toward patients; or become over-involved.4 Course content on these issues could be introduced early and developed further during clinical and postgraduate phases.
There are many opportunities pre-clinically to learn about the effects of abuse and trauma on human development, including the short and long term effects on the brain and behaviour. Relevant knowledge can be taught within sections of the curriculum devoted to neuroscience, cognitive science and population health. In addition, medical humanities have a powerful capacity to expand our knowledge and understanding of diverse human experiences, including trauma, and to foster empathy. Every clinical specialty that medical students and trainees encounter in hospital training brings opportunities to learn specialty-specific trauma knowledge and skills. For example, rotations in paediatrics and in obstetrics and gynaecology are opportunities for teaching about child abuse and about family violence.
Trauma is an everyday part of clinical discourse within psychiatry and a key dimension of academic and clinical learning in psychiatry rotations. However, trauma-informed education is relevant to all clinical specialties. Relevant specific knowledge encompasses common clinical presentations of trauma in those specialties; how trauma-relevant inquiry can be embedded within the specialty-specific clinical interview; clinical, social and legal services relevant to various forms of abuse; and legal requirements regarding mandatory reporting.
Some medical students and trainees have their own experiences of trauma, including childhood trauma and abuse, but also vicarious trauma stemming from clinical encounters, such as witnessing horrific physical injury or disfigurement. Post-traumatic stress disorder in medical practitioners is often unrecognised.14 Such experiences may increase or may impair empathic capacity to engage with traumatised patients.
Although the AMC standards discuss the stressful and traumatic nature of medical work and provide recommendations about availability of counselling, peer support and other measures, they construe the reality of trauma as external to the core business of medical education.13 Instead, we propose that learning about the emotional impacts of clinical work should be core medical education, to be dealt with in lectures, tutorials, simulations — that is, a range of appropriate, complementary educational methods, as is done for other topics. In addition, curricula should include safe, confidential, non-coercive opportunities for experiential learning in small groups, allowing participants to reflect on and share their own emotional reactions to patients and understand how these reactions can shape their clinical practice. Ideally, some teaching should be conducted jointly with students from other disciplines, notably nursing.
The AMC standards stress the centrality of clinical clerkships in the development of clinical competence and judgement.13 We agree, but when it comes to trauma-informed clinical teaching, there are several entailments. Teaching about trauma has to become a routine, everyday feature of clinical teaching in wards and clinics, not something outsourced by referral to psychiatry or social work. Clinician teachers in all specialties need to acquire the skills to do such teaching and to act as role models. Given the limited evidence base, it is premature to recommend a mix or staging of methods, and this should be a focus of future research and curriculum innovation.
The medical education literature is marked by separate discourses on differing forms of trauma. For example, education regarding intimate partner violence has been extensively explored and excellent curricula have been implemented.3,13,14 However, patients have often experienced several concurrent or sequential traumas; and the clinical sequelae of different traumas have many similarities, demand similar clinical skills (albeit allied with different bodies of specific knowledge), and thus present similar challenges for medical education. Valuable educational synergies are likely if the currently disparate, unconnected trauma-relevant elements in medical curricula are integrated.
These considerations point to the need for the creative design and evaluation of staged, incremental, integrated programs, structured to achieve continuity between undergraduate, pre-vocational and specialist phases of medical education. We do not propose removing the various trauma-specific educational components from medical curricula and replacing them with some form of generic trauma education. We do propose, however, examining creatively how they may be better integrated to become mutually reinforcing.
Conclusion
Trauma-informed health care is an invaluable concept which we propose should extend to trauma-informed medical education.15 Although the arguments for trauma-informed medical education are compelling, new lines of educational research will be needed to guide curriculum design and build on the small body of work already available.3,4,11,12,16 It is likely that if doctors of all kinds have the knowledge, skills and attitudes to deal competently with abuse and trauma, we can expect improvements in patient care and health service costs, and in the health and wellbeing of medical practitioners. These possibilities deserve empirical study. As well as becoming better clinicians, medical students and trainees will also become better teachers and role models and, as they move into more senior and leadership roles, advocates for competent trauma-informed medical care.
[World Report] As Hajj nears, concerns about public safety linger
Despite recent efforts to improve the sites of the Hajj pilgrimage in Mecca, some experts say that risks remain for visitors because of gaps in education and planning. Chris McCall reports.
Vocational training of general practitioners in rural locations is critical for the Australian rural medical workforce
The known In efforts to reduce the longstanding geographically inequitable distribution of Australian GPs, current policy requires that 50% of GP vocational training (registrar) positions are located in rural or remote areas.
The new We identified a strong association between rural training pathways and subsequent rural practice, and it is intensified by a rural origin effect. Despite some attenuation over time, these associations remained strong up to 5 years after vocational registration.
The implications Ongoing support for rural GP vocational training opportunities and the selection of rural origin medical students are critical components of GP workforce policy.
The geographically inequitable distribution of the Australian medical workforce continues, and rural and remote general practitioner positions are largely filled by international medical graduates (IMGs).1 This dependency persists despite substantial government efforts to stimulate recruitment and retention of Australian-trained GPs in rural areas. Recent government initiatives have included a large increase in the number of federally supported medical school places for students, and supporting medical education and training in rural communities through the Rural Clinical Training and Support (RCTS) program.1,2 A quota for the proportion of domestic students with a rural background selected by medical schools (at least 25%) has also been introduced, and rural clinical exposure during undergraduate and pre-vocational medical training programs has increased. In addition, Australian policy now requires that 50% of GP vocational (registrar) training occurs outside metropolitan areas.1 This policy is based chiefly on research that has indicated that a positive educational experience in rural settings, targeted training of GP registrars for rural practice, and clear pathways to rural practice are the most effective incentives for interesting a GP in a rural career.3,4 Doctors accepted into GP training are selected into either the Rural Pathway or the General (mostly metropolitan) Pathway, with about 50% of candidates allocated to each.5
Evidence for the effectiveness of these interventions for increasing rural recruitment and retaining Australian medical graduates in rural areas has accumulated. Ranmuthugala and colleagues6 reported that evidence for the effectiveness of increased rural exposure during undergraduate medical training on the uptake of rural practice was inconclusive, but Wilkinson and colleagues7 found that postgraduate rural GP training had a stronger association with rural practice uptake than rural exposure during undergraduate training (although the availability of rural GP postgraduate training was low at the time of this study because the number of rural training positions was limited). More recent empirical data8–10 and data on intentions collected at training completion11,12 suggest moderate improvement in the uptake of rural practice by students who have participated in RCTS programs. However, as reported in three literature reviews on the recruitment and retention of medical practitioners in rural areas3,13,14 and as lamented in a recent letter to the Medical Journal of Australia,15 there remains a large evidence gap as to the effectiveness of rural exposure during vocational training programs. A review of the outcomes of the regionalised Australian General Practice Training Program16 found that only 27% of former Rural Pathway registrars remained in rural practice after 7 years. In addition, several North American studies have produced limited quantitative evidence of associations between vocational training in a rural primary care setting and subsequent rural practice.17–20
The geographic origin of doctors also has an impact on their commencing rural practice, with convincing evidence about a strong link between an individual’s rural upbringing and their subsequent decisions about a rural career.21,22 The consistency of the reported association between GPs having a rural background and their choosing a rural career suggests that their origin is a critical factor in making this decision, regardless of vocational training location. Our study therefore aimed to investigate the association between vocational training location and the subsequent choice of practice location for newly registered GPs, including the effect of a rural background.
Methods
This study was based on data from the Medicine in Australia: Balancing Employment and Life (MABEL) study, conducted by the Centre for Research Excellence in Medical Workforce Dynamics (https://mabel.org.au/). MABEL is a national longitudinal survey that collects annual data from a panel of doctors, with a regular small participation top-up. The first wave of the MABEL study (2008) invited the entire medical workforce to participate, and 10 498 doctors (19.4% of the medical population) completed the initial survey, including 17.7% of GPs. There has subsequently been an annual 70–80% study retention rate. Further participants (generally recently graduated, non-specialist hospital doctors or IMGs newly registered in Australia) are added to the MABEL pool each year.
Our study analysed data from MABEL waves 1 to 7 (2008–2014), and was restricted to respondents who had completed their GP vocational training and were transitioning to independent practice. The transition year for a GP was identified from MABEL data on the basis of their participation in GP registrar training and details of newly completed medical qualifications. Data for IMG GPs — defined as those who had completed their initial medical training outside Australia and New Zealand — were analysed separately.
Rural origin and work location
Rural origin was defined for doctors trained in Australia or New Zealand as their having resided for at least 6 years in a rural area before the age of 18 years. Each doctor’s work location was geocoded in each MABEL wave to a specific town or suburb, then classified as metropolitan or rural. Rural location was defined as including Australian Standard Geographic Classification Remoteness Areas (ASGC-RA) 2 to 5;23 it was self-defined for New Zealand-trained doctors. Vocational training location was defined in two ways: as rural or metropolitan by work location in the year the doctor completed their training (final training location), and as an aggregate of work locations in the 2 to 3 years preceding their completion of training.
Statistical analysis
Four cohorts were defined by a combination of origin type and final training location: rural origin/rural training, metropolitan origin/rural training, rural origin/metropolitan training, and metropolitan origin/metropolitan training. For comparison purposes, IMGs were separately divided into two cohorts: rural training and metropolitan training.
A secondary (sensitivity) analysis defined four cohorts by multiple training locations: rural training only; completed training in a rural area, but also had some metropolitan training; completed training in a metropolitan area, but also had some rural training; metropolitan training only.
For each cohort, the proportions of GPs working in rural and metropolitan locations were calculated for each of the first 5 years after they had completed their vocational training. Rurally trained GPs were further classified according to whether they were working in the same or a different rural community from that in which they completed their vocational training; a buffer of 20 kilometres was allowed.
Separate generalised estimating equation (GEE) models with a logit link function and exchangeable correlation structure were used to test associations between vocational training pathways and subsequent work location for the four primary cohorts (non-IMGs only) for each of the 5 years after completing vocational training. Adjustments were made for four additional demographic variables during each particular year: sex, age, living with a partner, and having dependent children. A further variable — whether the GP was rurally bonded (contracted to work for part of their early career in rural locations) in a particular year — was included in each regression model. These models were repeated for the four secondary cohorts, with rural origin as an additional covariate; its multi-year cohort definitions limited analysis to 4 outcome years. All calculations were performed in StataSE 12 (StataCorp).
Ethics approval
The MABEL study was approved by the University of Melbourne Faculty of Business and Economics Human Ethics Advisory Group (reference, 0709559) and the Monash University Standing Committee on Ethics in Research Involving Humans (reference, CF07/1102 – 2007000291).
Results
During the 7-year study period, 610 doctors completed their GP vocational training and commenced in at least one subsequent work location. The demographic characteristics of these GPs are summarised in Box 1. Just under half of the local graduates (ie, those who graduated in Australia) trained in the Rural Pathway, and about one quarter were of rural origin (consistent with current policy requirements for GP training posts and medical student intakes); fewer than 10% were rurally bonded. Most local medical graduates were women, most lived with a partner, and almost 40% had dependent children. The proportions of IMGs who trained in the Rural Pathway, were men, were aged 35 years or more, lived with a partner, or had dependent children were higher than for local medical graduates (Box 1).
Box 2 summarises the practice location as an independent GP for the four primary cohorts of local medical graduates for each of the 5 years following their completion of vocational training. There were very strong and sustained associations between final vocational training location type and subsequent practice location for the rural origin/rural training and metropolitan origin/metropolitan training cohorts; 74–91% and 87–95% respectively remained in their origin/training type during their first 5 post-training years. Moreover, 61–70% of the rural origin/rural training cohort practised in the same rural community in which they trained during the first 4 years after completing their vocational training. Outcomes for GPs from cohorts 2 and 3 also showed a clear pattern: initially, these GPs generally remained in their final vocational training location type, but there was subsequently a gradual move in work location toward their origin type. The career patterns of rurally trained IMGs was similar to those of metropolitan origin/rural trained local graduate GPs, with a gradual move in work location toward metropolitan areas during the 5 years after vocational registration (Box 3).
The rural training pathway, regardless of childhood location, was highly significantly associated with subsequent rural practice. The odds of rural practice for each of the rural training cohorts of GPs decreased with time, but a strong and highly significant association was nevertheless retained across the 5 years. Unsurprisingly, rural bonding and rural origin were positively associated with rural practice. Higher age was also associated with rural practice, while there were no consistent statistically significant associations between practising in a rural location and sex, or with having a partner or dependent children (Box 4).
Secondary analysis, using the multiple year training location definition, confirmed the importance of rural training, particularly that of the final GP training year (Box 5).
Discussion
We have provided empirical evidence for the contribution of rural vocational training, in combination with the selection of rural origin students, to the Australian rural GP workforce. This is highly significant for rural workforce policy, as the Australian government requires that more than half of Australian GP vocational training positions be located in rural areas; our study allows an opportunity to assess the effect on the workforce of these policies.1
We found that training in the rural training pathway and the trainee having a rural background were each strongly associated with early career rural practice. The strength of the association between vocational training location and choosing rural practice remained strong and statistically significant up to 5 years after completing GP training for doctors of either rural or metropolitan origin (primary cohorts 1 and 2). Sustained rural practice was very strongly linked with the combination of a rural origin and rural training, but this cohort alone is unlikely to provide a sustainable rural GP workforce while only 25% of Australian-trained doctors are of rural origin, as about 30% of the Australian population live in rural or remote areas.
Most mixed rural/metropolitan origin/training GPs (cohorts 2 and 3) subsequently practised in a same location type as that in which they trained, although some gradually returned to their origin type. Diminution of the pathway effect over time is perhaps expected, as 50% of GP registrar training positions are in rural areas but about 75% of young doctors are of metropolitan origin. Other research has found that work location changes are most likely during early career stages,24 when personal circumstances, including relationships with spouses and dependents, are more fluid. The secondary analysis confirmed the strong influence of rural training on subsequent rural practice, especially location during the final year of vocational training. Together, these findings suggest that the periods leading up to and immediately following vocational training are critically important windows of opportunity for ensuring that appropriate policies optimise recruitment of GPs for rural practice and their subsequent retention.25,26
The largest cohort, metropolitan origin doctors undertaking GP training in metropolitan areas (cohort 4) largely remained in metropolitan practice. Further, there was no evidence that rural origin Australian doctors were more likely than metropolitan origin doctors to choose general practice as their specialty (unpublished MABEL data). Consequently, metropolitan origin doctors continue to remain the major source of non-IMG rural GPs, making cohort 2 (metropolitan origin/rural training) critical for the rural GP workforce. This cohort is nearly twice the size of cohort 1, and the association with rural practice was much stronger than for those in the metropolitan pathway (cohort 4). However, more than 50% of cohort 2 had moved to metropolitan practice after 5 years, further highlighting the importance of targeted retention initiatives focused on this cohort.
The odds of members of the smallest cohort (cohort 3: local medical graduates with a rural background who undertook their training in metropolitan areas) practising in rural areas was three times that for metropolitan origin/metropolitan training GPs, although the association was statistically significant only from 3 years after completing vocational training. However, the odds were much lower than for the rural origin/rural training cohort 1, highlighting the importance of the rural training pathway.
A key limitation of this study is that it cannot establish cause and effect. There is probably a strong self-selection bias, in that the rural training pathway attracts those who are interested in a rural career. Further limitations include the use of a self-selected cohort, the participants of the MABEL survey, who represent 15–18% of all Australian GPs. While the panel design of our study enabled individual tracking of doctors over a 7-year period and application of GEE (logit) modelling, the observed patterns, particularly in the mixed origin/training cohorts, suggest that these doctors have not yet decided on their long term preferred work location, and it is therefore difficult to accurately predict outcomes at, for example, 10 or 20 years. Additionally, vocational training location was primarily defined for the purposes of this study as the location of the trainee in the year they completed their training, as this was considered to be the most influential year for subsequent practice location. Our secondary analysis partially examined this aspect by separately analysing GPs who had undertaken vocational training in a mix of rural and metropolitan locations. Further, our key focus was on the joint effects of rural origin with rural/metropolitan training pathways. This necessitated a focus on GPs who had completed their medical degrees in Australia or New Zealand, despite IMGs comprising a considerable proportion of the rural GP workforce in Australia (more than 50% in some regions). Finally, this study used a binary measure of rurality (metropolitan v non-metropolitan) that may not adequately adjust for the substantial heterogeneity in the attractiveness to GPs of different rural and remote Australian locations. It is possible that more nuanced measures of rurality, including multiple levels of remoteness and population size, might have identified different associations for the four cohorts.27
Conclusion
Our study analysed the best available Australian longitudinal data about individual GPs to provide new quantitative evidence of a strongly positive association between rural GP vocational training location and subsequent rural practice, even after adjusting for the influence of rural origin. This evidence supports the objectives of existing policies that require at least 50% of GP training to occur in rural locations, and that at least 25% of medical students should be of rural origin. While Australia strives to reduce its reliance on IMG GPs for the rural workforce, this aim requires long term improvements in the rural recruitment and retention of Australian-trained GPs. Ongoing support for rural GP vocational training opportunities is a critical component of rural GP workforce policy in Australia.
Box 1 –
Demographic characteristics of participating doctors at the time they completed general practitioner vocational training
|
|
Local medical graduates |
International medical graduates |
|||||||||||||
|
|
|||||||||||||||
|
Number |
467 |
143 |
|||||||||||||
|
Rural Pathway (year of training completion) |
221 (47.3%) |
101 (70.6%) |
|||||||||||||
|
Rural origin |
118 (25.3%) |
NA |
|||||||||||||
|
Sex (women) |
322 (69.0%) |
74 (51.8%) |
|||||||||||||
|
Age, median |
32 years |
41 years |
|||||||||||||
|
Age, ≥ 35 years |
153 (32.9%) |
125 (89.9%) |
|||||||||||||
|
Living with a partner |
335 (72.7%) |
119 (83.2%) |
|||||||||||||
|
Has dependent children |
179 (39.4%) |
119 (83.8%) |
|||||||||||||
|
Rurally bonded |
35 (7.5%) |
NA |
|||||||||||||
|
|
|||||||||||||||
|
NA = not applicable. Percentages exclude missing data for local medical graduates (age, 2; living with partner, 6; dependent children, 13) and international medical graduates (age, 4; dependent children, 1). |
|||||||||||||||
Box 2 –
Final vocational training location and general practice location for local medical graduates during the first 5 years after completing general practitioner vocational training
|
Time since completion of training |
Location of practice |
(1) Rural origin/rural training |
(2) Metropolitan origin/rural training |
(3) Rural origin/metropolitan training |
(4) Metropolitan origin/metropolitan training |
||||||||||
|
|
|||||||||||||||
|
|
Number of GPs |
78 (17%) |
143 (31%) |
42 (9%) |
204 (44%) |
||||||||||
|
1 year |
Same rural |
70% |
54% |
— |
— |
||||||||||
|
Other rural |
20% |
22% |
18% |
5% |
|||||||||||
|
Metropolitan |
10% |
25% |
82% |
95% |
|||||||||||
|
2 years |
Same rural |
62% |
42% |
— |
— |
||||||||||
|
Other rural |
24% |
31% |
30% |
13% |
|||||||||||
|
Metropolitan |
14% |
27% |
70% |
87% |
|||||||||||
|
3 years |
Same rural |
68% |
24% |
— |
— |
||||||||||
|
Other rural |
15% |
42% |
35%* |
11% |
|||||||||||
|
Metropolitan |
18% |
34% |
65%* |
89% |
|||||||||||
|
4 years |
Same rural |
61% |
25% |
— |
— |
||||||||||
|
Other rural |
30% |
29% |
46%* |
9% |
|||||||||||
|
Metropolitan |
9% |
45% |
54%* |
91% |
|||||||||||
|
5 years |
Same rural |
42%* |
15% |
— |
— |
||||||||||
|
Other rural |
32%* |
33% |
33%* |
9% |
|||||||||||
|
Metropolitan |
26%* |
52% |
67%* |
91% |
|||||||||||
|
|
|||||||||||||||
|
* Groups with fewer than 20 participants. |
|||||||||||||||
Box 3 –
Final vocational training location and general practice location for international medical graduates during the first 5 years after completing general practitioner vocational training
|
Time since completion of training |
Location of practice |
Rural training |
Metropolitan training |
||||||||||||
|
|
|||||||||||||||
|
|
Number of GPs |
101 (71%) |
42 (29%) |
||||||||||||
|
1 year |
Same rural |
81% |
— |
||||||||||||
|
Other rural |
6% |
4% |
|||||||||||||
|
Metropolitan |
13% |
96% |
|||||||||||||
|
2 years |
Same rural |
57% |
— |
||||||||||||
|
Other rural |
17% |
8% |
|||||||||||||
|
Metropolitan |
26% |
92% |
|||||||||||||
|
3 years |
Same rural |
49% |
— |
||||||||||||
|
Other rural |
10% |
0* |
|||||||||||||
|
Metropolitan |
41% |
100%* |
|||||||||||||
|
4 years |
Same rural |
45% |
— |
||||||||||||
|
Other rural |
21% |
18%* |
|||||||||||||
|
Metropolitan |
34% |
82%* |
|||||||||||||
|
5 years |
Same rural |
53%* |
— |
||||||||||||
|
Other rural |
7%* |
20%* |
|||||||||||||
|
Metropolitan |
40%* |
80%* |
|||||||||||||
|
|
|||||||||||||||
|
* Groups with fewer than 20 participants. |
|||||||||||||||
Box 4 –
Odds of local medical graduates practising in a rural location during the first 5 years after completing general practitioner vocational training
|
|
Odds ratio (95% confidence interval) |
||||||||||||||
|
1 year post-GP training |
2 years post-GP training |
3 years post-GP training |
4 years post-GP training |
5 years post-GP training |
|||||||||||
|
|
|||||||||||||||
|
Primary cohorts |
|
|
|
|
|
||||||||||
|
(1) Rural origin/rural training |
159 (45–558)† |
65 (27–158)† |
48 (22–102)† |
50 (24–106)† |
52 (24–111)† |
||||||||||
|
(2) Metropolitan origin/rural training |
68 (26–175)† |
32 (16–60)† |
28 (16–51)† |
23 (13–41)† |
24 (13–43)† |
||||||||||
|
(3) Rural origin/metropolitan training |
2.8 (0.7–11) |
2.4 (0.9–6.2) |
2.9 (1.2–6.7)* |
3.3 (1.5–7.4)† |
3.5 (1.5–7.9)† |
||||||||||
|
(4) Metropolitan origin/metropolitan training |
1.00 |
1.00 |
1.00 |
1.00 |
1.00 |
||||||||||
|
Age (for each 1-year increase in age) |
1.06 (1.00–1.13)* |
1.04 (0.99–1.08) |
1.04 (1.00–1.08)* |
1.05 (1.01–1.08)* |
1.04 (1.01–1.08)* |
||||||||||
|
Sex (reference: men) |
1.00 (0.48–2.1) |
0.9 (0.5–1.6) |
1.03 (0.6–1.7) |
0.8 (0.5–1.4) |
0.8 (0.5–1.4) |
||||||||||
|
Living with a partner |
0.8 (0.3–1.9) |
0.9 (0.5–1.7) |
0.9 (0.5–1.7) |
0.98 (0.6–1.7) |
0.9 (0.6–1.5) |
||||||||||
|
Has dependent children |
1.8 (0.8–4.1) |
1.9 (1.06–3.3)* |
1.4 (0.9–2.3) |
1.3 (0.9–2.0) |
1.3 (0.9–1.9) |
||||||||||
|
Rurally bonded |
5.1 (1.2–22)* |
3.5 (1.1–11)* |
3.8 (1.4–11)* |
3.7 (1.4–10)* |
3.6 (1.3–10)* |
||||||||||
|
|
|||||||||||||||
|
Odds ratios from generalised estimating equation (logit) model: * P < 0.05; † P < 0.01. |
|||||||||||||||
Box 5 –
Odds of practising in a rural location for each of the 4 years after completing general practitioner training for local medical graduates
|
|
Odds ratio (95% confidence interval) |
||||||||||||||
|
1 year post-GP training |
2 years post-GP training |
3 years post-GP training |
4 years post-GP training |
||||||||||||
|
|
|||||||||||||||
|
Secondary cohorts |
|
|
|
|
|||||||||||
|
(1) Rural training only |
92 (27–312)† |
49 (21–115)† |
41 (19–88)† |
29 (14–59)† |
|||||||||||
|
(2) End training rural, with some metropolitan training |
17 (5–58)† |
11.6 (4.6–29)† |
11.5 (4.9–26)† |
9.9 (4.3–23)† |
|||||||||||
|
(3) End training metropolitan, with some rural training |
0.94 (0.09–9.4) |
2.8 (0.8–9.4) |
2.9 (1.00–81) |
2.7 (0.96–7.9) |
|||||||||||
|
(4) Metropolitan training only |
1.00 |
1.00 |
1.00 |
1.00 |
|||||||||||
|
Rural origin |
4.1 (1.3–13)* |
2.0 (0.9–4.3) |
2.1 (1.02–4.1)* |
2.5 (1.3–4.9)† |
|||||||||||
|
Age (for each 1-year increase in age) |
1.2 (1.04–1.3)† |
1.08 (1.01–1.16)* |
1.07 (1.01–1.14)* |
1.05 (1.00–1.12) |
|||||||||||
|
Sex (reference: men) |
0.9 (0.3–2.4) |
0.8 (0.4–1.7) |
0.9 (0.5–1.9) |
0.8 (0.4–1.5) |
|||||||||||
|
Living with a partner |
0.6 (0.2–2.1) |
1.1 (0.5–2.6) |
1.1 (0.5–2.4) |
1.07 (0.5–2.1) |
|||||||||||
|
Has dependent children |
0.6 (0.2–2.0) |
1.3 (0.6–2.7) |
1.09 (0.6–2.1) |
1.02 (0.6–1.8) |
|||||||||||
|
Rurally bonded |
2.0 (0.4–10) |
2.21 (0.6–7.8) |
3.8 (1.2–13)* |
3.6 (1.1–11)* |
|||||||||||
|
|
|||||||||||||||
|
Odds ratios from generalised estimating equation (logit) model: * P < 0.05, † P < 0.01. |
|||||||||||||||
Rural recruitment and training promotes rural practice by GPs, but is it enough to retain them?
Challenges to keeping general practitioners in the bush remain
The findings reported by McGrail and colleagues in this issue of the MJA support the effectiveness of Australian government incentives for recruiting and training general practitioners in rural areas as a strategy for reducing rural medical workforce shortages.1 The study found that rural origin of trainees and rural vocational training of GPs were each strongly associated with their practising in rural areas in the early years after completing vocational training. However, their findings also suggest that these effects had started to diminish by 4 years post-training.1 This finding is consistent with another recent Australian study, which found that the effects of rural recruiting and training diminished over time.2
As evidence emerged in the early 1990s that a rural background and a positive rural training experience promoted the subsequent uptake of rural practice by trainees, the Australian government introduced several initiatives for recruiting and training medical students in rural areas. The Rural Undergraduate Support and Coordination Program (RUSC) was in 1993 among the first of these initiatives, followed by the Rural Clinical School (RCS) and the Rural Clinical Training and Support Program (RCTS). These initiatives required that 25% of the intake of students by federally funded medical schools be from a rural background; that all federally supported medical students undertake a 4-week structured rural placement; and that 25% of students undertake at least 12 months’ clinical training in a rural location.3 Initiatives such as the Australian General Practice Training Program followed, ensuring that at least 50% of general practice vocational training placements are in rural or remote areas.4 These training initiatives have contributed to the success achieved in increasing the number of GPs who adopt rural practice: it was recently reported that the rural and remote GP workforce increased by 23% between 2010 and 2014, compared with a 3.5% increase in the rural and remote community population, and a 10% increase in the metropolitan GP workforce over the same period.5
It is now timely to consider whether an increase in the number of rural and remote GPs necessarily translates into a sustained and well supported workforce which can deliver quality health care that meets the needs of rural communities. Factors that motivate practitioners to remain in rural areas include access to training, professional development and career development opportunities.3 While I focus in this article on the role of training and education in rural retention, other factors known to be important include peer and professional support, assistance with heavy workloads and on-call requirements, locum relief,3 access to infrastructure (such as information and communication technology and electronic health data systems), housing, and family support.6
In addition, being a principal of the medical practice has been identified as significantly increasing the likelihood of a doctor remaining in a rural location (by 72%), while being a salaried or contracted employee significantly reduces the likelihood (by 20–30%).7 GPs in rural and remote locations work longer hours than their metropolitan counterparts, increasing steadily from an average of 38 hours per week in metropolitan locations to 45.8 hours in very remote locations.5 Such demands, and the need to travel, make it more difficult for rural or remotely located practitioners to participate in professional development and to take up training opportunities. Innovative business and work model solutions are needed to support the rural GP workforce.
It should also be noted that the proportion of GPs practising procedural skills increases with remoteness (from 8.0% in inner regional areas to 13.8% in outer regional and 20.9% in remote and very remote locations).5 Recognising that rural and remote practitioners must have procedural skills in general surgery, obstetrics, anaesthesia, radiology and endoscopy, the Royal Australian College of General Practitioners has incorporated procedural skills training into their curriculum.8 Additional training is provided through the General Practitioner Procedural Training Support Program. Nevertheless, the period 2010–2013 saw a drop in the proportion of GPs practising procedural skills;5 the decline was greatest in outer regional areas (4.1%), followed by remote (3.9%), inner regional (1.9%) and very remote locations (0.6%). Reasons for this decline are not clear and need further exploration, especially given a recent finding that undertaking hospital work significantly increases the likelihood that rural and remote GPs remain in rural locations (by up to 40%).7 As exercising one’s skills contributes to increased job satisfaction, motivation, commitment and retention,9 there is a need to provide the infrastructure and opportunity for these practitioners to enhance and practise the procedural skills that have been identified as an important aspect of rural practice.
The early training initiatives are having positive effects on recruitment, but they must be reviewed and updated as new evidence emerges. Accordingly, in light of consistent support for the influence of longer term rural clinical placements on the likelihood of choosing rural practice, the initial requirement that all federally supported medical students undertake a 4-week rural placement has been reduced to 50% of students, but with no change to the proportion required to undertake a year-long rural clinical placement.10 It will be another 5–10 years before the effect of these revised funding parameters on the recruitment and retention of the rural medical workforce will be apparent.
The potential of workplace-based assessment of international medical graduates
The concept is attractive — but capacity may limit its practicality
For many years, Australia has relied on supplementing its medical workforce with doctors who have qualified outside Australia. Each year, about 2500 of these medical practitioners, known as international medical graduates (IMGs), seek general registration with the Medical Board of Australia. For many IMGs, this has included sitting the clinical examinations conducted by the Australian Medical Council (AMC) as part of the Standard Pathway for IMGs. The eligibility standard for registration is set at the expected level of an Australian medical graduate at the time they complete their internship.1 Concerns have been expressed about the accessibility of these examinations and the ability of IMG candidates to pass them. Some of these problems were highlighted during an inquiry in 2011–2012 by the House of Representatives’ Standing Committee on Health and Ageing, Lost in the labyrinth.2
Workplace-based assessments (WBAs) have been developed as alternatives to the Objective Structured Clinical Examination (OSCE) and other approaches for assessing clinical competence.3 They have the perceived advantage of allowing a single set of high stakes (summative) assessments in an examination environment to be replaced by multiple low stakes (formative) assessments conducted by supervising clinicians over a period of time and in the workplace. These methods have been progressively adopted in recent years by medical specialist colleges. The AMC commissioned a trial of WBAs as an alternative to their clinical examination in 2010, and have subsequently incorporated WBAs into the Standard Pathway.
An article by Nair and colleagues in this issue of the MJA evaluates the reliability of WBAs in this context.4 The usefulness of an assessment method relies on a number of psychometric criteria being fulfilled. These include the concepts of validity (does the assessment method reflect performance in practice?), reliability (is it reproducible and consistent?), feasibility (is it an efficient use of resources?), acceptability, and educational impact.5 WBAs are generally recognised as having high validity, as they are conducted in the workplace where the doctor is practising and are modelled on and executed as part of normal clinical practice.
Reports have previously been published about the feasibility and acceptability of WBAs for assessing IMGs, and on the reliability of a single method, the mini-clinical evaluation exercise (mini-CEX).6–8 A combination of assessment methods, however, allows different aspects of clinical practice to be assessed.3 The article by Nair and colleagues examined the reliability of such a combination (“composite reliability”), and found that it was good (reliability coefficient greater than 0.8).
Why is this important? In an examination process, reliability can be ensured by standardised processes, similar formats, and controlling the examiners, candidates and the examination environment. However, this often compromises the validity of the examination. The increased validity associated with WBAs, on the other hand, can affect reliability because of variations and pressures in the clinical environment, the fact that examinees are dealing with real patients, differences in assessment formats, and the lesser control over the candidates and assessors. A high degree of reliability for a combination of assessments indicates that candidates are being assessed in a standard and reproducible manner and to an equivalent standard of competence, comparable with the standard AMC examination process. The results reported by Nair and colleagues are, however, for a single program; for these findings to be generalised to other WBA programs for IMGs, further research may be required.
What do these results mean for assessing IMGs in the future? In 2015, 84 candidates participated in the WBA program, and 76 completed it successfully. In comparison, changes to the AMC processes and the establishment of the Vernon C. Marshall National Test Centre in Melbourne have allowed 2000 candidates to attempt the clinical examination over the same period (with about 590 completing it successfully).9 While WBAs have been shown to be feasible, affordable and reliable, they require resourcing and a commitment from the host institution. Further, candidates need to be recruited to a training position in a hospital offering the program. Investing in the program has rewarded some hospitals with improved recruitment and retention of practitioners, which is important in regional areas where this can be difficult. However, competition for training positions has increased; there are more than 3000 domestic medical graduates each year who need pre-vocational training.10
The AMC clinical examination offers access to a greater number of IMGs (possibly reflected in the lower rate of successful completions than for WBA). Australia continues to be an attractive destination for medical graduates from other countries, and the demand for assessment will therefore continue. WBAs will be a useful part of that assessment, but there are limits to the number of candidates who can be accommodated by this approach, especially when compared with the AMC examination.

 more_vert
more_vert