Minor surgery is an important aspect of general practice. This is particularly the case in Australia, where the incidence of skin cancer is reported to be the highest in the world,1 and where general practitioners perform most surgical excisions for skin cancer.2
When the use of gloves for surgery was first implemented by William Stewart Halsted in 1890, it was in an attempt to protect his surgical scrub nurse from dermatitis as a result of contact with mercuric chloride — which was used for sterilisation processes — rather than to prevent infection.3 Nowadays, several guidelines exist in Australia and internationally, which recommend that GPs use sterile gloves for small procedures such as minor surgery in general practice.4–6 However, these guidelines are based on expert opinion rather than on medical evidence.
Before our study, about half of the participating GPs used non-sterile clean boxed gloves when conducting minor skin excisions in general practice, while the other half used sterile gloves. A comprehensive Medline search found few studies relating to the use of sterile versus non-sterile gloves (Appendix). Randomised trials looking at lacerations in an emergency department,7 wisdom tooth extraction in an outpatient setting8 and Mohs micrographic surgery9 all showed no significant difference between infection rates. However, these studies looked for superiority of the sterile gloves rather than non-inferiority of the non-sterile gloves, resulting in negative trials, and the latter two studies were statistically underpowered. An observational study in a private dermatology setting showed no difference in infection rate for minor procedures; however, sterile gloves were shown to result in a significantly lower infection rate than non-sterile gloves for a subgroup of more complicated reconstructive procedures, which comprised flaps and skin grafts.10 Another observational study of Mohs surgery showed no statistical difference in infection rates.11 The only study conducted in a general practice setting was an audit of 126 patients where non-sterile gloves had been used for minor surgery, which showed an infection rate of 2.4%.12
Prior studies of wound infection after minor surgery involving GPs in Mackay, Queensland, showed overall incidences of wound infection of 8.6% and 8.9%.13–16 This incidence was higher than expected based on published results of a similar Australian general practice cohort (1.9%),17 a skin cancer clinic cohort (1.5%)18 and a European dermatology clinic cohort (2%).19 A suggested acceptable rate of infection after clean minor surgery is less than 5%.20 The reason for our high infection rate is unclear, but may be related to the hot, humid environment, or to patient behaviour in our rural setting. A low risk of infection after clean surgery means that studies of more than 1000 procedures (sometimes many more) are required, under normal circumstances, to detect a clinically relevant difference in infection from an intervention with statistical confidence.21 Because of the high incidence of infection in our patient cohort, and the high minor surgery workload,22 we decided to use this capacity to investigate the effect of gloves on infection rates. Our trial sought to establish whether non-sterile clean boxed gloves were non-inferior to sterile gloves with regard to surgical site infection after minor skin excisions.
Methods
Study design
We carried out a randomised controlled single-centre trial with patients presenting for minor skin excisions. The study was approved by the James Cook University Human Research Ethics Committee (approval number H4572). The trial was registered in the Australian New Zealand Clinical Trials Registry ACTRN12612000698875.
Setting and participants
The study was conducted in a single private general practice in Mackay, Queensland between 30 June 2012 and 28 March 2013. Six doctors recruited between one and 100 patients. The GPs and practice were purposively selected as they had previously successfully participated in wound management projects.12,15 Consecutive patients presenting for minor skin excisions were invited to take part in the trial. Practice nurses were responsible for recruiting patients and collecting data. Demographic information was collected from all patients, as well as clinical information about diabetes or any other important pre-existing medical conditions. A body site map was used to define excision site. At the end of the study, practice nurses were asked to re-examine computer records in order to fill in any missing data. Two of us (C H and S S) visited participating GPs and practice nurses to provide training and ensure that recording was standardised.
Eligibility criteria
All patients presenting to a participating GP for “minor skin excision” from any body site were eligible to participate in the study. Two-layer procedures were recorded and included. Patients who were already taking oral antibiotics or immunosuppressive drugs were excluded from the study. Other exclusion criteria were skin flaps, excision of a sebaceous cyst and history of allergy to latex.
Surgical wound management protocol
We conducted a workshop for participating GPs to develop guidelines that would ensure that excisions were managed in a standardised manner. The following excision protocol was agreed on:
- skin preparation with chlorhexidine solution;
- usual sterile technique (standard precautions);
- World Health Organization Hand Hygiene Technique with Soap and Water;23
- local anaesthesia — subcutaneous injection of excision with 1% lignocaine;
- excision closure with nylon sutures using simple interrupted sutures;
- dressing application — application of non-woven polyester fabric with acrylic adhesive and non-woven absorptive pads;
- no application of antibiotics, either topical or oral. No topical antiseptics such as betadine or alcohol. No antiseptic washes or medicated soaps;
- patient wound advice — provision of written and verbal advice about wound care and time of return for suture removal; and
- removal of sutures according to body site: head and neck, 7–10 days; torso, 12–14 days; upper limb, 14 days; lower limbs, 12–16 days.
Recruitment, randomisation and blinding
All patients provided written informed consent before enrolling in the study. After agreeing to participate, patients were randomly allocated to the intervention or control groups using computer-generated random numbers. Allocation information was placed in opaque sealed envelopes. The practice nurse enrolled patients and assigned participants to their groups. Patients were not blinded to their group allocation. The assessing practice nurses and doctors were blinded to the allocation of intervention and control groups. All participating patients received written instructions on postoperative wound care. Both groups were asked to take their dressing off after 24 hours and avoid using antiseptics.
Clinical outcomes
Incidence of wound infection was our primary outcome measure, and incidence of other adverse effects was our secondary outcome measure. Wounds were assessed for infection by the practice nurse or the GP on the agreed day of removal of sutures or sooner if the patient re-presented with a perceived infection. Our definition of wound infection was adapted from standardised surveillance criteria for defining superficial surgical site infections developed by the United States Centers for Disease Control and Prevention’s National Nosocomial Infection Surveillance System (Box 1).24 All participating doctors and nurses were briefed regarding the definition of infection and were also given written information. Practice nurses were asked to swab any discharging infections to investigate any pattern of antimicrobial resistance.
Sample size
Sample size was calculated on the basis of our previous study, which showed an infection rate of 8.6%.14 Based on a projected infection rate of 8%, we decided that an absolute increase in incidence of infection of 7% would be clinically significant. Thus differences in infection rates between non-sterile and sterile gloves of up to 7% were considered clinically unimportant, and based on our anticipated infection rate of 8% for sterile gloves, an infection rate of up to 15% for non-sterile gloves was considered non-inferior. This margin was decided by the investigating GPs, based on what they felt would be relevant to their clinical practice, and this margin was prespecified. To detect this non-inferiority margin of 7% with a power in excess of 80%, and a two-sided 95% confidence interval, a total of 186 patients were required in the intervention group and 186 patients in the control group. Based on our previous results in a similar setting, the design effect of investigating GPs, who were the primary sampling unit and were considered to form “clusters”, was estimated to be 1.21, and the required sample size was adjusted to at least 225 patients per group.16
Statistical analysis
All analyses were based on the intention-to-treat principle. Per-protocol analyses were conducted to cross-validate the intention-to-treat results.25,26 Depending on the distribution, numerical data were described as mean, SD; or median, interquartile range (IQR). Percentages were presented with 95% confidence intervals. A two-sided 95% CI for the difference in infection rate was used to assess non-inferiority. In addition, a per-protocol analysis was conducted, which excluded patients with protocol violations. Further, a sensitivity analysis was performed, including patients lost to follow-up: once as treatment successes (no wound infection) and once as treatment failures (with wound infection). Results were adjusted for their cluster effects. P less than 0.05 were considered statistically significant. Data were analysed using IBM SPSS version 21, Stata version 12.1 (StataCorp) and Power Analysis and Sample Size Software (NCSS).
Results
Practice and study characteristics
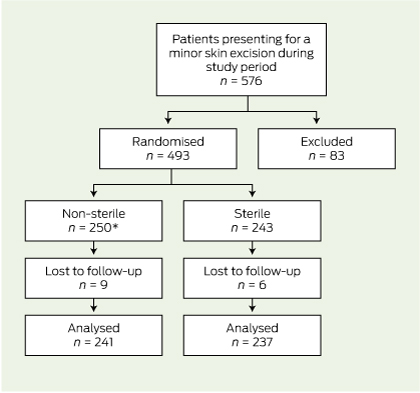
Of the 576 patients who attended for skin excisions during the collection period, 83 were excluded (Box 2).
Of the remaining 493 patients, 250 were randomly assigned to the intervention group (non-sterile gloves) and 243 to the normal treatment control group (sterile gloves). Fifteen patients were eventually lost to follow-up because they had their sutures removed elsewhere (13 patients) or they were not assessed for infection at the time of removal of sutures (two patients). There was one protocol violation where a patient in the intervention group was given an antibiotic for another infection in the follow-up period. This patient did not have a wound infection and was analysed in the intervention group on an intention-to-treat basis. Follow-up was completed in 478 (97.0%) of randomised patients (Box 3).
Comparisons at baseline
There were no large differences at baseline between the intervention and control groups (Box 4).
Incidence of infection
Infection occurred in 43 of the 478 excisions (9.0%). The incidence of infection in the non-sterile gloves group (8.7%; 95% CI, 4.9%–12.6%) was significantly non-inferior compared with the incidence in the control group (9.3%; 95% CI, 7.4%–11.1%). The two-sided 95% CI for the difference in infection rate (− 0.6%) was − 4.0% to 2.9%, and did not reach the predetermined margin of 7%, which was required for non-inferiority.
A further sensitivity analysis was performed on the 15 patients lost to follow-up. If all of these patients were assumed to have an infection, or if all patients were assumed not to have an infection, the results were still significantly non-inferior (Box 5). There were no adverse events.
Discussion
The results of our study suggest that the use of non-sterile clean boxed gloves was not inferior to that of sterile gloves in relation to the incidence of infection. This was both clinically and statistically significant, as the difference in the incidence of infection did not reach our predetermined margin of 7%, considered significant for non-inferiority. The upper limit of our 95% CI was 2.9%, which was well below our predetermined non-inferiority margin of 7.0%.
Comparison with other studies
Our study produced a similar outcome to existing studies.7–9 This was an adequately powered, positive randomised controlled trial that tested for non-inferiority of the non-sterile gloves rather than for a significant difference in infection rates. We believe this was the first study of its type to be conducted in a general practice setting.
Limitations of study
Our study did have some limitations. Various characteristics influence infections, and although information on as many variables as possible was recorded, it proved difficult to ensure that baseline data were comparable. For example, there were inadequate data recorded on suture size and patient occupation, and consequently, these factors could not be compared. In addition, the prevalence of diabetes and other medically important conditions was probably underrecorded, and power to analyse these subgroups was limited. Surgical training and technique of the GPs involved is a potential confounder that would be difficult to quantify and was not recorded; however, the procedures performed by individual GPs were equally balanced in the baseline data. Our predetermined margin of 7% for non-inferiority may be considered high, and some clinicians may consider a smaller margin to be clinically meaningful. Although our actual difference in infection was − 0.6%, a larger sample size would be required for the study to be adequately powered to detect smaller differences in infection rate.
Although the diagnosis of infection followed guidelines, it is still subjective and there may be inter- and intraobserver variation.27 The definition we used is the most widely implemented standard definition of wound infection.24,27 We have no evidence to support intra- and interpractice reproducibility of measurement and recording procedures.27
Our sterile gloves were powdered, while our non-sterile gloves were non-powdered. However, we have no reason to believe that powder would affect infection rates.
Generalisability
There are some limits to generalising these findings. The population of Mackay is slightly older and has a lower median household income than the general Australian population.28 Mackay is a provincial town in tropical north Queensland. The climate is hot and humid, with the mean daily maximum temperature ranging between 24.2°C and 30°C during the summer months, and a relative humidity of 75% to 79%.29 We have already discussed that our incidence of wound infection is high compared with similar cohorts of patients in temperate climates; however, we have no reason to believe that the effect of sterile gloves would be less non-inferior, that is, any worse, in similar cohorts of patients with lower incidence of surgical site infection.
We did not include skin flaps in our trial, and previous evidence has shown sterile gloves to be superior for more reconstructive dermatological procedures;10 therefore, we do not recommend extrapolating our findings to more complicated procedures such as skin flaps. However, the findings could be extrapolated to less complicated procedures in primary care, such as contraceptive implant insertion and minor procedures involving class 2 wounds such as suturing of lacerations.
Choice of gloves
There are other considerations that might affect doctors’ choice of gloves. Sterile gloves come in several different sizes, while non-sterile gloves are generally only available in small, medium and large. Latex and powder allergy, as well as preference for and availability of powdered or non-powdered gloves, may also affect choice. A recent study showed high bacterial counts on boxed gloves left open for longer than 3 days,30 although the clinical significance of these bacterial counts is unclear. Another study showed no bacterial growth on clean examination gloves after opening a new box.31
Cost saving
There is some cost benefit in the use of non-sterile versus sterile gloves, with about $1 saved per pair of gloves used. We calculated that a single pair of non-sterile gloves costs $0.153 compared with $1.203 for sterile gloves, saving $1.050 per pair of gloves used for each procedure. The cost saving benefit of using non-sterile gloves — without increasing infection rates — may be of particular relevance to developing countries with limited health care resources.
1 Definition of surgical site infection (SSI)
- infection must be within 30 days of excision;
- the infection involves ONLY skin or subcutaneous tissue of the incision, AND at least one of the following:
- purulent discharge;
- pain or tenderness;
- localised swelling;
- redness or heat at site;
- diagnosis of SSI by general practitioner; and
- stitch abscess must not be counted as an infection.
2 Reasons for exclusion from study
|
Reasons for exclusion from study
|
Patients (n = 83)
|
|
|
Patient declined to participate
|
38
|
|
Patient was taking oral antibiotics
|
23
|
|
Excision of sebaceous cyst
|
15
|
|
Shave biopsy conducted
|
3
|
|
Patient did not plan to return for removal of sutures
|
2
|
|
No sutures required
|
1
|
|
Flap required
|
1
|
3 Flowchart of enrolment, randomisation and follow-up of patients
4 Baseline comparison of intervention group (non-sterile gloves) and control group (sterile gloves)
|
Patient characteristics
|
Intervention group (non-sterile gloves) (n = 241)
|
Control group (sterile gloves) (n = 237)
|
|
|
Mean age (SD), years
|
64.9 (15.8)
|
65.7 (15.3)
|
|
Male
|
58.9%
|
60.30%
|
|
Smoking status
|
|
|
|
Never smoked
|
57.7%
|
52.7%
|
|
Ex-smoker
|
30.7%
|
35.9%
|
|
Current smoker
|
11.6%
|
11.4%
|
|
Diabetes mellitus
|
10.0%
|
12.7%
|
|
Other medical conditions*
|
38.1%
|
35.9%
|
|
Medications
|
|
|
|
Warfarin
|
4.1%
|
5.1%
|
|
Clopidogrel or aspirin
|
28.6%
|
27.0%
|
|
Steroids, oral or inhaled
|
6.3%
|
8.1%
|
|
Lesion characteristics
|
|
|
|
Body site
|
|
|
|
Neck and face
|
35.3%
|
31.2%
|
|
Upper extremities
|
26.9%
|
30.4%
|
|
Trunk
|
19.1%
|
19.8%
|
|
Lower limb above knee
|
4.6%
|
1.6%
|
|
Lower limb below knee
|
14.5%
|
16.9%
|
|
Histology
|
|
|
|
Naevus or seborrhoeic keratosis
|
15.3%
|
13.0%
|
|
Skin cancer and precursor†
|
66.4%
|
70.5%
|
|
Other‡
|
18.3%
|
16.5%
|
|
Skin integrity
|
|
|
|
Normal
|
75.9%
|
74.7%
|
|
Ulcerated
|
19.1%
|
19.0%
|
|
Procedure characteristics
|
|
|
|
Mean length of excision (SD), mm
|
20.0 (14.0–27.0)
|
20.0 (13.5–27.0)
|
|
Median number of days until removal of sutures (IQR)
|
8 (7–10)
|
9 (7–10)
|
|
Two-level procedure
|
0
|
0.8%
|
|
|
IQR = interquartile range. * Medical conditions recorded were: chronic obstructive pulmonary disease (n = 18; 3.8%), hypertension (n = 119; 24.9%), ischaemic heart disease (n = 38; 7.9%), peripheral vascular disease (0) and current cancer (n = 7; 1.5%). † Skin cancers were: melanoma, squamous cell carcinoma and basal cell carcinoma. Precursors were: solar keratosis and intra-epithelial carcinoma. ‡ “Other” included: re-excisions of melanoma and basal cell carcinoma, sebaceous cyst, epidermal cyst, wart and dermatitis.
|
5 Comparisons as intention-to-treat and per-protocol and sensitivity analyses*
|
Analysis
|
Intervention group
|
Control group
|
Difference
(95% CI)
|
|
|
Intention-to-treat
|
21/241 (8.7%)
|
22/237 (9.3%)
|
− 0.6%
(− 4.0% to 2.9%)
|
|
Per-protocol
|
21/240 (8.8%)
|
22/237 (9.3%)
|
− 0.5%
(− 4.0% to 2.9%)
|
|
Sensitivity analysis: lost to follow-up; assumed without infection
|
21/250 (8.4%)
|
22/243 (9.1%)
|
− 0.7%
(− 4.0% to 2.7%)
|
|
Sensitivity analysis: lost to follow-up; assumed with infection
|
30/250 (12.0%)
|
28/243 (11.5%)
|
0.5%
(− 3.7% to 4.6%)
|
|
|
* Differences between control and intervention groups are presented with two-sided 95% confidence intervals. Results were adjusted for the clustering effects of treating doctors.
|

 more_vert
more_vert