Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia, affecting more than 33·5 million individuals worldwide.1 Despite pharmacological advances in the past two centuries, digoxin remains the oldest and one of the most common adjunctive treatments for rate control in AF. Although reports show a persistent decrease in overall digoxin prescription rates,2 its use remains common in contemporary AF trials in nearly a third of participants.3
Preference: Cardiology and Cardiac Surgery
99
Chronic Q fever prosthetic valve endocarditis — an important cause of prosthetic valve dysfunction in Australia
Clinical record
An 86-year-old man, a retired structural engineer, was referred to our tertiary centre with a 3-week history of New York Heart Association Class III–IV heart failure symptoms. Past medical history included bioprosthetic aortic valve (23 mm Perimount) implanted 10 years previously for severe aortic stenosis due to age-related degeneration, ischaemic heart disease (coronary artery bypass grafting in 2003), chronic kidney disease stage 2, dyslipidaemia, and previous smoking. He had been closely followed by his local cardiologist with slowly progressive dysfunction of the aortic valve replacement, thought to represent prosthetic valve degeneration, which was asymptomatic and managed conservatively. Importantly, there were no symptoms or clinical suspicion of chronic endocarditis. A sudden clinical deterioration, with clinical signs of left ventricular failure, prompted a referral to hospital.
On examination, the patient was haemodynamically stable and afebrile. There were no peripheral stigmata of infective endocarditis. His jugular venous pressure was raised at 5 cm, and he had lower limb pitting oedema. Bibasilar crackles were noted on chest auscultation, and there were murmurs consistent with mixed prosthetic valve stenosis and regurgitation. The patient’s abdomen was soft, with no evidence of hepatosplenomegaly.
Laboratory investigations showed a white blood cell count of 10.2 × 109/L (reference interval [RI], 3.5–11.0 × 109/L), a haemoglobin level of 120 g/L (RI, 120–180 g/L) and a platelet count of 149 × 109/L (RI, 140–400 × 109/L). Chest x-ray showed cardiomegaly.
Transthoracic echocardiography showed severe prosthetic valve aortic regurgitation and thickening of the prosthetic valve leaflets with moderate aortic stenosis, with a maximum gradient of 96 mmHg and a mean gradient of 51 mmHg. Transoesophageal echocardiography confirmed severe transvalvular aortic regurgitation and thickened prosthetic valve leaflets, but no defined vegetations were present (Box 1).
Three sets of blood samples were cultured, the results of which were all negative. Cardiac catheterisation showed native triple vessel coronary disease, a patent left internal mammary arterial graft and mildly diseased but patent venous grafts.
Based on the patient’s presenting signs and echocardiography findings, it was concluded that repeat aortic valve replacement was indicated. His case was discussed at the multidisciplinary heart valve team meeting to decide between valve-in-valve transcutaneous aortic valve implantation (TAVI) and redo sternotomy and surgical aortic valve replacement. The consensus was for repeat cardiac surgery with a redo bioprosthetic aortic valve.
The patient’s condition was stabilised medically, and he underwent a redo sternotomy and transverse aortotomy. Macroscopic assessment of the explanted bioprosthetic aortic valve showed significant structural deterioration, with calcified central nodularity on all three leaflets surrounded by erythema, and destruction of leaflet tissue (Box 2). A 23 mm Perimount valve (Edwards Lifesciences) was secured in place. He developed atrial fibrillation after surgery, which was managed medically. The patient’s recovery was otherwise uncomplicated, and he was discharged home 8 days after surgery.
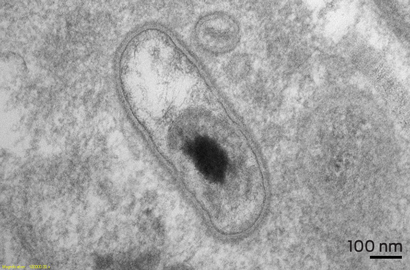
Pathological examination of the specimen showed marked nodular fibrosis and calcification. Preliminary microscopy and cultures of operative specimens revealed no microorganisms. However, transmission electron microscopy of tissue sections confirmed intracellular organisms, in keeping with Coxiella burnetii chronic active endocarditis (Box 3 and Box 4).
The patient and his general practitioner and cardiologist were immediately informed, and the patient was commenced on 18 months’ doxycycline and hydroxychloroquine therapy, under advice from the infectious diseases team.
Q fever serological investigations subsequently showed antiphase I IgG titre of 3200 (normal titre, < 800), which represented chronic Q fever. Further detailed history-taking ruled out any known Q fever infection in the past, contact with farm animals or consumption of unpasteurised milk. However, he lives in a regional area of Australia, which is endemic for C. burnetii.
The bacterium C. burnetii is a small, obligate intracellular gram-negative organism that was first described by Derrick in Australia in 1937.1 Q fever is a zoonosis transmitted from its primary reservoirs, usually farm animals, to humans mainly via inhalation but also by consuming unpasteurised dairy products.2 C. burnetii infection can affect the liver, spleen, skin, lungs, kidneys and central nervous system. Cardiac manifestations include culture-negative endocarditis, myocarditis and pericarditis.2 Presenting symptoms are usually non-specific and protean, making the diagnosis, particularly of chronic Q fever, challenging.
Q fever endocarditis (QFE) is one of the main causes of culture-negative endocarditis.3 Degenerated and damaged native aortic or mitral valves are most commonly targeted by C. burnetii.2 Prosthetic valves and prior valve surgery also predispose to QFE.4
The diagnosis of QFE is usually based on serological investigations, bacterial cultures and polymerase chain reaction (PCR) testing. Echocardiography may detect valvular vegetation in only 30% of cases.5
QFE is a potentially fatal disease if not diagnosed and treated in time.5 It has been recommended that patients with prosthetic valves but without infective endocarditis who are successfully treated for acute Q fever should have serological follow-up every 4 months for 2 years.6 In the setting of QFE, due to risk of relapse even after successful treatment, it is recommended that patients should have serological follow-up for at least 5 years.7
This case provides a unique learning point that QFE of a prosthetic valve (QFE-PV) can present as silently progressive, asymptomatic prosthetic valve dysfunction. In the past 5 years, 13 patients with progressive bioprosthetic valve dysfunction were found to have infective endocarditis at redo surgery at the Prince Charles Hospital in Brisbane, Queensland; two of these cases were due to QFE-PV. Clinicians should have a low threshold for investigation of QFE-PV in patients with prosthetic heart valves living in a region endemic for Q fever.
A second issue is the performance of Q fever serological investigations before consideration for TAVI. If TAVI were to occur in the context of unsuspected C. burnetii infection, the infection is likely to go undetected and result in early degeneration of the prosthesis from recurrent endocarditis.
In conclusion, QFE-PV is an unsuspected cause of prosthetic valve dysfunction, but may be present in a proportion of patients requiring redo surgery. QFE-PV should be suspected in patients with degenerative heart valve replacements living in C. burnetii endemic countries such as Australia. Increased physician awareness of this condition, and early performance of C. burnetii serological investigations for any patient with early prosthetic valve dysfunction (regurgitation or stenosis), is recommended.
Lessons from practice
- Q fever endocarditis of a prosthetic valve can result in premature prosthetic valve dysfunction among patients with cardiac valve replacements.
- Clinicians should have a low threshold to exclude Q fever endocarditis of a prosthetic valve in a patient with prosthetic valve dysfunction who lives in a Q fever endemic region.
- After successful treatment of acute Q fever endocarditis, regular follow-up is required to detect and treat chronic Q fever.
1 Transoesophageal echocardiograph of the aortic valve prosthesis in the short axis orientation
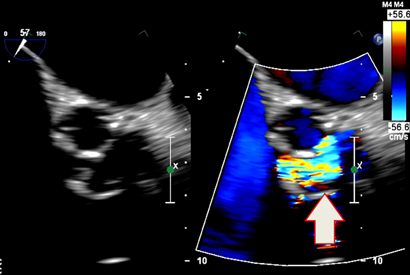
Thickening of the leaflets is visible on the left, and a central zone of severe aortic regurgitation is visible on the right (arrow).
2 Macroscopic surgical specimen of the excised bioprosthetic valve
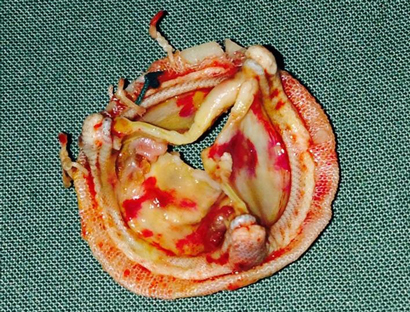
Nodular thickening of the leaflets and destruction of the leaflet tissue are visible on the specimen.
Better prevention and management of heart failure in Aboriginal Australians
Striking disparities in heart failure incidence and outcomes warrant urgent attention
Heart failure (HF), a common sequel of many cardiovascular diseases and predisposing risk exposures, remains a major health problem despite recent advances in medical therapy.1 HF in Aboriginal Australians is characterised by a substantial and distressingly familiar excess in incidence (Box),2 morbidity and mortality, particularly at younger ages.2–4
Strategies to enhance the prevention and early detection of heart failure
The primary prevention of HF in Aboriginal people is paramount and must occur concomitantly with treatment efforts. This requires population-based approaches to address underpinning social determinants of health5 (eg, poverty, marginalisation, environmental factors); cardiovascular risk factor reduction (smoking, obesity, diabetes, hypertension, dyslipidaemia); increased physical activity; and early detection and management of structural heart disease. Prevention must include multisectoral strategies to address the structural–systemic factors that undermine Aboriginal people’s opportunities throughout life. Additionally, screening, monitoring and treatment of HF antecedents in the community setting are needed to reduce HF incidence further, with suitably trained Aboriginal people central to delivering culturally appropriate health education. Further, primary care health professionals should be trained to detect subclinical HF using echocardiography6 and to manage predisposing risk factors optimally.
National guidelines highlight the importance of best-practice management for secondary prevention of HF comprising accessible, multidisciplinary, evidence-based and patient-centred care to improve HF outcomes.1,7 Yet the management of HF in practice remains problematic, with poor continuity of care and fragmented service provision, particularly for Aboriginal people. Rehospitalisation with HF is common, with the majority of readmissions triggered by potentially modifiable factors like poor discharge planning, inadequate competency for self-management and non-adherence to medications and diet, exacerbated by delays in medical consultation for escalating symptoms.8 Shortcomings are amplified for Aboriginal patients, who have additional challenges from increased HF severity; comorbidities; socioeconomic, language and cultural barriers; reduced access to cardiac rehabilitation; and limited access in rural and remote areas.1,2,4–6 A more coordinated but flexible, patient-focused approach that takes into account the complex patient journey of Aboriginal patients, particularly those from rural areas,9,10 is required to enhance chronic HF care for Aboriginal Australians.
Strategies to improve the management and outcomes in patients with clinical heart failure
Although the evidence for successful programs to manage HF and other chronic diseases in Aboriginal Australians is currently inadequate, several studies are underway that will provide a better evidence base to guide this.10–12 While engaging Aboriginal people throughout, implementation of the following actions should occur now, with refinement as new evidence emerges.
- Provide all Aboriginal patients hospitalised with HF, regardless of where they live, with a coordinated, seamless, patient-centred pathway of care. This requires clear clinical protocols and support using Indigenous care coordinators and health workers.
- Enhance the Aboriginal cultural competency of organisations and service providers.
- Strengthen primary health care services and Aboriginal voices within service delivery through engagement of communities.
- Integrate service and program delivery between mainstream services and Aboriginal community-controlled primary health care services, encouraging robust and effective partnerships.
- Recruit Aboriginal champions to create greater awareness of HF and its risk factors.
- Encourage Aboriginal people to enter health careers and enhance support to health professionals in rural and remote areas to address inadequate staffing and workforce turnover.
- Improve health literacy and HF management by providing culturally appropriate information on HF prevention and treatment to the Aboriginal community and individual caregivers.13
- Ensure effective clinical information systems (eg, patient medical records, recall systems) and protocols for information flow and sharing to improve hospital discharge planning, transition to community with appropriate support that includes Aboriginal care coordinators, and ongoing specialist oversight.
- Use technology to overcome geographical challenges, enhancing the use of telemedicine and remote monitoring to improve access to enable specialist input.14
- Use modern and appropriate technology for reminders of follow-up visits and medications.
- Develop and support comprehensive cardiac rehabilitation programs (incorporating education, psychosocial support, exercise training, optimal pharmacotherapy) with ongoing case management.10,15
- Ensure sufficient discharge medications to cover patients’ return home and next review; and involve community pharmacists and health workers for reminders and checks.
- Change or enhance traditional models of care to incorporate family-based and outreach programs, taking into account clinical, logistical and cultural complexity.9,10
- Facilitate the involvement of Aboriginal people in research and education.
Systematic delivery of multidisciplinary, patient-centred care for Aboriginal Australians with HF is crucial for improving health outcomes, especially for those living in rural and remote areas. This requires rethinking traditional models of care delivery. Importantly, we must ensure that Aboriginal Australians with HF experience satisfactory quality of life and are engaged with their family in end-of-life care decisions. Much progress has occurred with injection of new funds through the Closing the Gap initiatives. Commitment to long-term strategies is critical.
Incidence of first heart failure hospitalisation in Aboriginal compared with non-Aboriginal patients, by age group and sex

Adapted from Teng et al.2
Estimating the current and future prevalence of atrial fibrillation in the Australian adult population
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia observed in medical practice. It ranges in severity, from isolated and benign episodes of electrical disturbance to a chronic cardiac condition that results in cardiac remodelling and functional impairment. Typically, AF progresses from paroxysmal to more permanent forms, irrespective of management practices and intervention, leading to a significant and independent risk of thromboembolism, cardiac failure and mortality.1,2 AF also adversely affects patients’ quality of life.3
AF has substantial economic impact, particularly due to AF-attributable stroke, the incidence of which is increasing in parallel with ageing populations as treatment remains suboptimal.
The evolving burden of AF has been influenced by a combination of population ageing, changing patterns of cardiac risk factors and improved survival rates in other, contributory forms of cardiovascular disease.1–5 As such, reports from high-income countries have demonstrated that AF exerts a major and evolving public health, social and economic burden.
Previously, the overall population prevalence of AF (in all age groups) was reported to be 1.0% to 2.0%.6,7 Recently, prevalence has been shown to be 2.5% to 4.0% in adult populations ≥ 18 years.8–10 Prevalence of 3.8% in adults ≥ 60 years has been described, with AF not commonly reported in individuals aged < 55 years (prevalence 0.1%).7
Much of the current literature describing AF epidemiology reports on statistics calculated from data derived from North American and European populations6,7,11–14 and, more recently, from Asian populations.15 The number of affected individuals is projected to increase exponentially over the next four decades.7 Large population-based epidemiological studies assessing the burden of AF in Australia are yet to be undertaken. Of significance, a recent analysis of national data in Australia confirmed a 7.9% annual increase in hospitalisations due to AF (as a principal diagnosis), with around 45 000 hospitalisations attributable to AF per annum.16 These data provide further impetus to understand the underlying caseload of AF in Australia.
The primary objective of our study was to estimate the current prevalence of AF in Australian adults most commonly affected by AF (those ≥ 55 years) using robust and reliable population data and international prevalence statistics. A secondary objective was to further estimate the likely prevalence of AF in Australia over the next two decades to 2034 as the population continues to age and the natural history of AF, and therefore its prevalence, remains unchanged.
Methods
Applying international AF prevalence statistics
We have previously undertaken a comprehensive review of the epidemiology of AF in high-income countries.8 For our current analysis, we assessed seven international epidemiological studies identified in the review and collated statistics to obtain estimates of AF prevalence that would be most applicable to the Australian population.6,7,11–15 We used data from studies that provided the most comprehensive age- and sex-specific prevalence estimates (including 95% confidence intervals) for individuals aged ≥ 55 years. We included studies with a prospective study design that presented more recent data for relatively large samples of relative similarity to the Australian population and with a rigorous method for definitive diagnosis of AF (ie, confirmed by electrocardiography). The Rotterdam Study11 most explicitly fitted these inclusion criteria.
Estimating current prevalence of AF in Australia
We obtained data representing the Australian population by single year of age as calculated on 30 June 2014 from the Australian Bureau of Statistics (ABS).17 Data for all individuals aged ≥ 55 years were combined into 5-year age brackets (except those aged ≥ 85 years, who were treated as one group). This was undertaken for the overall population and for each state and territory separately. We applied international AF prevalence statistics to each age group and to men and women separately. Ethics approval was not required for this research, as it involved the use of publicly available anonymised data and did not directly involve human participants.
Projecting future prevalence of AF in Australia
We obtained population projections for 2014 to 2034 from the ABS based on the last census (2011) and representing annual data as at 30 June. These projection data represent the growth and change in the Australian population that would occur if certain assumptions about future fertility, mortality and migration were to prevail over the projection period.18 Projections for the total population and by sex were condensed into 5-year age groups (for people ≥ 55 years). Conservatively, we assumed that AF prevalence would remain stable from 2014 to 2034 and, therefore, the same prevalence statistics were applied. Three main series of projection data were available for use. We chose the series that largely reflects current trends in fertility, life expectancy at birth and net overseas migration.18 We also undertook a sensitivity analysis in which the upper and lower limits of prevalence CIs were applied to all data (Appendix 1).
Results
Estimated prevalence of AF in 2014
The estimated prevalence of AF in Australian adults aged ≥ 55 years at 30 June 2014 was 5.35% (95% CI, 3.79%–7.53%; Box 1) and was greater in men than in women (5.97% [95% CI, 4.11%–8.54%] v 4.79% [95% CI, 3.50%–6.60%], respectively). However, reflective of global patterns, the number of prevalent cases of AF among women aged > 80 years is likely to be greater than that among age-matched men. With the exception of men aged 70–74 years and women aged 75–79 years, there was a consistent trend towards more AF cases in successively older age cohorts. Of note, our estimates suggest that among 60–64-year-olds there are currently 2.5 times as many men as women with AF, and among those aged 65–69 or 75–79 years, there are almost twice as many men as women with AF.
Appendix 2 shows the estimated prevalence of AF and case distribution within each Australian state and territory at 30 June 2014. New South Wales was expected to have the highest number of individuals affected by AF overall (110 892), and the Northern Territory was expected to have the lowest (1413).
Projected prevalence of AF in 2034
We estimated that by 2034 AF prevalence will have increased by 1.04% in the overall adult population aged ≥ 55 years (to 6.39%; 95% CI, 4.56%–8.90%). This comprises an increase of 1.25% in men and 0.85% in women, to 7.22% (95% CI, 4.99%–10.28%) and 5.64% (95% CI, 4.18%–7.64%), respectively (Box 1 and Appendix 3). Thus, owing to population dynamics alone (ie, without any changes in incidence and survival rates), the number of individuals affected by AF in Australia will have almost doubled compared with 2014 — overall, from over 300 000 to over 600 000, and for men and women, respectively, from about 175 000 and 150 000 in 2014 to about 325 000 and 280 000 in 2034 (Box 1 and Appendix 3).
About double the number of individuals aged ≥ 75 years will be affected by AF by 2034 (414 377 compared with 200 638). Similarly, the number of affected men aged ≥ 85 years is predicted to increase by 2.5-fold (from 29 370 to 71 582).
Appendix 2 also shows the projected number of affected individuals and prevalence of AF within each Australian state and territory in 2034. It is estimated that NSW will still have the highest number of individuals with AF (191 578), a 1.7-fold increase, but Queensland will have the greatest increase (from 61 613 to 123 142; twofold). All Australian states and territories are projected to have between 1.7–2.3 times the number of individuals with AF.
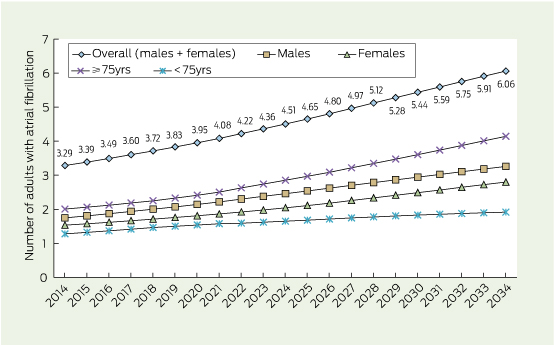
A steady increase in AF prevalence predicted between 2014 and 2034
As shown in Box 2, the number of adults aged ≥ 55 years with AF is predicted to increase steadily in Australia over the next two decades. The number of individuals aged 55–74 years with AF is projected to rise at a slower rate than the number of affected individuals aged ≥ 75 years. The difference between the estimated numbers of men and women with AF in 2014 is expected to continue and perhaps widen over time.
Discussion
Despite global recognition of an increasing burden of AF within the ageing populations of high-income countries,8,19 there is a paucity of data to quantify the number of Australians affected by this potentially deadly condition. Using contemporary population data and conservative estimates derived from robust international studies, we estimated that 5.35% of the adult population aged ≥ 55 years (5.97% of men and 4.79% of women) are currently affected by AF.
Projected rise in Australia
This proportion is projected to rise to about 1.2 times this value by 2034, to 6.39%. We predict that between 2014 and 2034 AF will continue to be more prevalent in older age groups and in men compared with women. Of all states and territories, NSW (being the most populous state) will experience the greatest increase in the number of cases (almost doubling, to just under 200 000), although the greatest increase in prevalence is expected in Queensland, most likely due to the ageing of an older resident population.
Overall prevalence of AF has risen substantially over recent decades, as shown by European, North American and Asian studies;6,7,11–15 however, there have been no contemporary large-scale, population-based studies conducted to determine AF prevalence in Australia. The last prospective Australian study collecting data specifically on the burden of AF was conducted in Western Australia between 1966 and 1981.20 Therefore, the contemporary epidemiological profile and cost burden of AF in Australia cannot be specifically described.
Global observations
Data from the our report contribute to exploring the size of the AF burden, reflecting global observations of prevalence, including sex and projection patterns. Reported AF prevalence is higher in older individuals, illustrating the influence of ageing; and prevalence in men remains consistently higher than in women, although absolute numbers of affected women are greater.
Our recent pooled analysis of international population data suggests that the prevalence figure (in the adult population ≥ 20 years) is likely to be between 2.5% and 3.5%,8 supporting the prevalence estimates presented here. Studies have confirmed that the prevalence of AF in community-based studies ranges between 0.1% and 4.0%9 in individuals aged ≥ 15 years and that overall prevalence of AF in Sweden was 3.0% (3.4% in men and 2.6% in women) in 2010.10 The two previous studies assessing AF prevalence in Australia demonstrate consistency with the conservative international estimates applied within our study, providing support for their use within the Australian context. Prevalence was reported to be 4.9% in WA adults ≥ 60 years (5.6% in men, 4.2% in women) between 1966 and 198120 and 4.0% in adults ≥ 30 years overall (6.0% in men, 4.0% in women) attending general practice in 2000.21 Our projections suggesting a pronounced escalation in AF cases in the coming decades match similar projections for European and North American populations.7,19
Perhaps the greatest impact of escalating AF prevalence will be economic. AF is associated with a five- to sevenfold increased risk of stroke and a threefold increased risk of heart failure.22 Within an ageing population, most costs will be driven by the need to manage stroke risk (via thromboprophylaxis), prevent cardiac dysfunction and manage patients after events secondary to AF (via hospitalisation, provision of therapy and increased surveillance). Although current Australian projections predict that prevalent AF cases will almost double in 20 years, others have predicted doubling over 25–35 years in North America.7,23 This differential is likely reflective of Australian population growth over the next two decades that will be shaped by increased international migration, urbanisation and population mobility.
Limitations
Some specific limitations of our work require comment. In order of importance: first, it is unknown whether the international prevalence statistics obtained from the Rotterdam Study are applicable to the Australian population and what the implications are of applying them. AF is known to be more prevalent in individuals of European ancestry,8 so the applied statistics may not translate to the increasingly multicultural Australian population. The prevalence of AF in Australian Aboriginal and Torres Strait Islanders remains unknown, but is potentially higher than in white Australians due to the increased cardiovascular disease burden within this population.24 Consequently, the selection of these international prevalence statistics may result in the over- or underestimation of prevalence within this population.
Second, increasing disease awareness, potential introduction of broad screening programs, and the ageing population will influence the incidence of AF. For example, single time-point AF screening identified an overall incidence of previously unknown AF of 1.0% (1.4% in those ≥ 65 years), of whom 67% were at high risk of stroke.25
Next, projections must be interpreted with some caution, given that certain factors may negatively or positively affect AF incidence and survival rates (fundamental drivers of prevalence). The current impact of antecedents and comorbidities of AF (eg, hypertension, obesity, obstructive sleep apnoea, diabetes, acute coronary syndrome) may change over time owing to increasing prevalence or improved therapies and management, suggesting that projections may potentially under- or overestimate prevalence, depending on the factors considered. The true prevalence of asymptomatic and paroxysmal forms of AF also remains unknown, potentially resulting in prevalence underestimation.
Finally, research will lead to acquisition of new knowledge on the natural history, treatment and progression of AF; for example, the development and introduction of novel therapeutics for management, which will have positive influences on survival. Assumptions were made when population projections were calculated; most critically, we assumed that AF prevalence remained static over time. Next, we chose population projections that largely reflect current trends in fertility, life expectancy and migration. We did not determine low and high estimates founded on alternative assumptions.
Conclusion
Our data provide a snapshot of the potential current and future impact of AF in Australia. They support the expectation that AF will reach epidemic proportions worldwide in the coming decades. Large-scale, population-based studies are required to ascertain the true burden of AF, including social, health and economic (direct and indirect) costs. Understanding this burden is premised on the availability of high-quality epidemiological studies. The increasing prevalence of AF will greatly influence health care systems and communities. Therefore, substantial integrated efforts to understand the complex causes and clinical presentation of AF in Australia are required to attenuate the large economic, social and individual costs associated with this potentially fatal cardiac condition.26
1 Australian adult population (aged ≥ 55 years) and estimated prevalence of atrial fibrillation (AF) at 30 June 2014
|
Australian population, 30 Jun 2014* |
Assumed prevalence,† % (95% CI) |
Population with AF at 30 Jun 2014, No. (95% CI) |
|||||||||||||
|
Age (years) |
(a) Men |
(b) Women |
(c) Total |
(d) Men |
(e) Women |
(f) Total (i ÷ c) |
(g) Men (a × d) |
(h) Women (b × e) |
(i) Total (g + h) |
||||||
|
|
|||||||||||||||
|
55–59 |
704 608 |
722 388 |
1 426 996 |
0.80% (0.30%–2.10%) |
0.60% (0.20%–1.50%) |
0.70% (0.25%–1.80%) |
5637 (2114–14 797) |
4334 (1445–10 836) |
9971 (3559–25 633) |
||||||
|
60–64 |
623 635 |
639 713 |
1 263 348 |
2.60% (1.60%–3.40%) |
1.00% (0.50%–2.00%) |
1.79% (1.04%–2.69%) |
16 215 (9978–21 204) |
6397 (3199–12 794) |
22 612 (13 177–33 998) |
||||||
|
65–69 |
554 463 |
563 844 |
1 118 307 |
5.20% (3.70%–7.30%) |
2.90% (1.90%–4.40%) |
4.04% (2.79%–5.84%) |
28 832 (20 515–40 476) |
16 351 (10 713–24 809) |
45 183 (31 228–65 285) |
||||||
|
70–74 |
400 375 |
417 265 |
817 640 |
6.90% (5.00%–9.60%) |
5.40% (4.10%–7.00%) |
6.13% (4.54%–8.27%) |
27 626 (20 019–38 436) |
22 532 (17 108–29 209) |
50 158 (37 127–67 645) |
||||||
|
75–79 |
288 732 |
321 994 |
610 726 |
13.00% (9.80%–17.10%) |
6.50% (4.70%–8.90%) |
9.57% (7.11%–12.78%) |
37 535 (28 296–49 373) |
20 930 (15 134–28 657) |
58 465 (43 430–78 030) |
||||||
|
80–84 |
195 863 |
252 391 |
448 254 |
15.20% (10.50%–21.50%) |
12.70% (9.70%–16.50%) |
13.79% (10.05%–18.68%) |
29 771 (20 566–42 111) |
32 054 (24 482–41 645) |
61 825 (45 048–83 756) |
||||||
|
≥ 85 |
164 078 |
291 302 |
455 380 |
17.90% (11.50%–26.80%) |
17.50% (13.80%–21.90%) |
17.64% (12.97%–23.67%) |
29 370 (18 869–43 973) |
50 978 (40 200–63 795) |
80 348 (59 069–107 768) |
||||||
|
Total |
2 931 754 |
3 208 897 |
6 140 651 |
5.97% (4.11%–8.54%) |
4.79% (3.50%–6.60%) |
5.35% (3.79%–7.53%) |
174 986 (120 357–250 370) |
153 576 (112 281–211 745) |
328 562 (232 638–462 115) |
||||||
|
|
|||||||||||||||
|
* Australian Bureau of Statistics population trends.17 † Based on international AF prevalence statistics. |
|||||||||||||||
Availability of highly sensitive troponin assays and acute coronary syndrome care: insights from the SNAPSHOT registry
Recent community campaigns on the warning signs of a heart attack have significantly increased the number of patients presenting to emergency departments (EDs) with possible acute coronary syndrome (ACS).1 Concomitantly, the advent of assays with improved sensitivity for detecting circulating cardiac troponins (cTn) — which are fundamental to the diagnosis of acute myocardial infarction (AMI) — has allowed the detection of low concentrations of these biomarkers,2 and an increase in proportions of ED patients with AMI has been reported.3
There has also been an associated increase in the number of patients with an elevated cTn level that is not attributable to an unstable coronary artery plaque resulting in an ACS.3 It is recommended that patients in the ED who have an elevated troponin level have further assessment and/or management of the underlying cause, which often includes admission to hospital with resulting use of resources.4 Thus, there is continued debate among clinicians about the overall utility of these highly sensitive assays, and questions about the overall population benefits.
The diagnosis of myocardial infarction (MI) requires a characteristic dynamic profile of myocyte necrosis of cardiac biomarker(s), preferably a troponin (troponin I and T).2 There are many assays for detecting these troponins, and these vary in analytical performance. There are two defining characteristics of a highly sensitive assay — it must have a coefficient of variation (a measure of analytical imprecision) of less than 10% at the 99th percentile of a healthy reference population, and should reliably measure results accurately in at least 50% of such a population.5 A scoring system has been proposed for assays, whereby highly sensitive assays should all be deemed “guideline acceptable”.5,6
The SNAPSHOT ACS study,7 a prospective audit of the management of consecutive patients admitted with suspected ACS during a 2-week period in Australia and New Zealand, allowed a unique opportunity to assess the real-time implications of the assays used. At the time of the audit, some centres in Australia and New Zealand had access to an assay with higher sensitivity (Roche hsTnT), while many continued to use the fourth generation cTnT assay or one of various cTnI assays. In this study we aimed to explore differences in the investigation, treatment, diagnosis and inpatient clinical course for patients whose management included the use of highly sensitive versus other troponin assay results.
Methods
The SNAPSHOT ACS study was conducted from 14 to 27 May 2012 in Australia and New Zealand, and has previously been described in detail.7 Briefly, it was developed as a collaborative quality improvement initiative between the Cardiac Society of Australia and New Zealand, the Heart Foundation of Australia, the Australian Commission on Safety and Quality in Health Care, the George Institute for Global Health, and health networks or state governments across Australia. Hospital participation, study protocols, recruitment, data collection and ethics approvals have been previously published.7
Over the audit period, consecutive patients having a first admission with a suspected or confirmed ACS were included. Patients were classified by primary discharge diagnosis into one of five diagnostic categories: (i) ST-segment-elevation MI/left bundle branch block (STEMI/LBBB); (ii) non-STEMI (NSTEMI); (iii) unstable angina and ischaemic chest pain; (iv) unlikely ischaemic chest pain, and (v) other diagnosis.
Each site recorded the troponin assay being used (including the troponin — I or T), and the upper limit of its reference interval (clinical decision cut-point). The Roche hsTnT assay (Roche Elecsys Troponin T with the clinical decision cut-point of 14 ng/L) has improved precision and meets the guideline-acceptable criteria.5,6,8 At the time of the study this was the only assay with higher precision in use in the two countries, and we refer to it in this study as the hs (highly sensitive) assay. The analytical profile of all other assays could collectively be termed sensitive or contemporary assays which were grouped into a single category termed “sensitive” assays. Hospitals that reported using both troponin T and troponin I assays where the upper limit of the reference interval for the troponin T assay was consistent with the hs assay cut-point of 14 ng/L were classified as having the hs test available.
Patients were grouped according to the binary troponin assay classification (hs assay or sensitive assay) that was used at the enrolling hospital. Patients who were subsequently transferred were analysed according to the assay used at the enrolling institution.
A common case record form was used for collating patients’ characteristics, recommended therapies for ACS and inhospital events, and logistical details of patient transfers between hospitals, and hospital resources were obtained.7
Inhospital events of death, new or recurrent MI, stroke, cardiac arrest or worsening heart failure were predefined (Appendix 1). Formal adjudication of events was not possible, but 2%–5% of all case record forms were monitored for data accuracy and quality by coordinators across all jurisdictions. Irrespective of the assay type, in accordance with the universal definition of AMI,2 a change in troponin level was required to define the diagnosis of NSTEMI (Appendix 1).
Statistical analysis
We present patient characteristics, investigations, and therapies among patients surviving to hospital discharge, and inhospital events. These are tabulated as unadjusted descriptive statistics stratified by troponin assay type (hs or sensitive) in the entire patient population and among those with a final diagnosis of ACS.
As indicators of the time to care, we compared the time to angiography and the overall length of stay between hospitals performing hs assays and sensitive assays. Because these data were highly skewed, we present unadjusted comparisons only.
Two main methods were used to attempt to yield an unbiased estimate of major cardiac event risk by type of assay. One involved using propensity score matching (producing an analysis subset of 3106 successfully matched patients) and a generalised estimating equation (GEE) to evaluate the association between assay type and outcome, accommodating hospital-level clustering. Within this analysis, a propensity score of likelihood for having care provided by a hospital that had the hs assay available was developed; this included age, sex, diagnosis, Global Registry of Acute Coronary Events (GRACE) risk score, prior MI, prior coronary artery bypass grafting (CABG), atrial fibrillation, prior stroke, peripheral vascular disease, prior renal disease, prior cancer, prior dementia, need for assistance with activities of daily living, residence in a nursing home, and Australian Institute of Health and Welfare (AIHW) hospital classification. Patients from a hospital with hs assays available were matched to those in a hospital without hs assays within a ± 2% difference in propensity score, yielding a cohort of 3106 patients (1545 and 1559 patients in the hs and sensitive troponin assay groups, respectively).
The second method involved an inverse probability-weighted (IPW) model with regression-adjusted estimators to estimate the averages of the predicted outcomes among patients who were treated with and without the availability of an hs assay for troponin in all patients. In this model, clinical and hospital variables used to estimate the probability of troponin assay type included diagnostic classification, GRACE score, glomerular filtration rate < 60 mL/min/1.73 m2, a history of diabetes and prior MI alongside metropolitan location of the hospital, an onsite cardiac service and catheter laboratory, and AIHW hospital classification. The association between troponin assay type and outcome was then further adjusted for the clinical variables described above, although no clustering on enrolling hospital was possible in this model.
A P value of < 0.05 was considered statistically significant and, as this was an exploratory analysis, no adjustment was undertaken for multiple comparisons. Analyses were undertaken using Stata version 13 (Statacorp).
Results
A total of 4398 patients were enrolled in 286 hospitals; data on troponin assay type was available for 4371 patients from 283 hospitals. Of these 283 hospitals, 156 (55%) used an hs troponin assay (Appendix 2). Most patients presented to hospitals that used the hs assay, of which 46 (29%) were principal referral hospitals or hospitals in major cities, with 21 (13%) being private hospitals (Appendix 2). There was no difference in the likelihood of use of hs assays compared with sensitive assays in institutions where cardiac services, including primary percutaneous coronary intervention, (PCI) were possible.
The characteristics of patients presenting to hospitals according to the assay type used are shown in Appendix 3. Patient discharge diagnoses according to the type of assay used are shown in Box 1.
Box 2 shows the use of resources for patients tested with the two groups of assays. Patients who presented to a hospital using an hs assay underwent more non-invasive investigations (eg, exercise stress tests). However, there was no difference between hospitals using the two groups of assays in the rates of angiography, PCI or CABG. Higher rates of angiography and PCI were observed among patients with a final diagnosis of ACS, and these differences persisted within the propensity-matched models (odds ratio [OR] for angiography, 1.58; 95% CI, 1.16–2.14; P = 0.003, and OR for PCI, 1.32; 95% CI, 1.0–1.73; P = 0.043)
Inhospital treatment for the cohort diagnosed with ACS varied in terms of the medications prescribed. Significant differences in the use of guideline-recommended therapy4 were observed, specifically in treatment with angiotensin-converting enzyme inhibitors and β-blockers (Box 2).
Box 3 shows that 442 patients had inhospital major adverse cardiac events; this represented 9.1% of patients in hospitals using an hs assay and 11.7% of patients in hospitals using a sensitive assay (P = 0.005). Using the two approaches (GEE and IPW) to assessing the association between assay type and outcome showed a consistently lower rate of inhospital events, including recurrent heart failure, in patients where an hs assay was used. By the GEE approach, the use of an hs troponin assay was associated with an odds ratio of 0.75 (95% CI, 0.60–0.94; P = 0.014). Similarly, by the IPW analysis, we estimated an event rate of 11.2% in patients in hospitals using sensitive troponin assays, with an average effect of a 2.3% absolute reduction in these events in patients in hospitals using the hs assay (P = 0.018).
Exploring the factors potentially influencing differences in outcome, we observed a shorter median delay in time to angiography in hospitals where the hs assay was available (38 hours; interquartile range [IQR], 14–72 hours for the hs assay v 43 hours; IQR, 19–75 hours for the sensitive assay; P = 0.0136), but no difference in total length of stay (2.5 days; IQR, 1.1–4.8 days for the hs assay v 2.6 days; IQR, 1.1–4.9 days for the sensitive assay; P = 0.43).
Discussion
The SNAPSHOT ACS registry provides several important insights into the use of highly sensitive troponin assays in the care of patients with suspected ACS.
Patients with suspected ACS who were cared for in hospitals using the hs troponin assay had a lower proportion of NSTEMI and a higher proportion of non-cardiac chest pain as final diagnoses. This contrasts with research cohorts where re-adjudication of diagnoses of individual ED patients showed an increased rate of diagnosis of NSTEMI when more highly sensitive assays were used.3 This observation may be explained by our study including more patients with suspected ACS who eventually had an alternative diagnosis (eg, arrhythmia, heart failure and pulmonary disease). The SNAPSHOT registry found a lower proportion of patients diagnosed with unstable angina in sites that used the hs assay. As elevated troponin levels are required for a diagnosis of AMI,2 this finding is not surprising. However, this is the first time that the magnitude of effect of troponin assays on AMI diagnoses has been reported in an Australian and New Zealand population.
There was no pattern in the type of institution that had access to the hs assay despite the perception that large metropolitan hospitals and private facilities would have greater access to the newer assays. Patients tested with hs assays had higher rates of non-invasive investigations than those tested with the other assays. There was no difference between hospitals using the sensitive or hs assay in terms of having cardiac intensive care, echocardiography or PCI services onsite. Hospitals using the hs assay did not have higher rates of angiography or PCI, consistent with no increase in the proportion of patients with ACS as their final diagnosis. Therefore, use of the hs assay may well drive a higher rate of some investigations, including non-invasive tests.
A significant finding was that, for patients at hospitals using the hs assay, inhospital rates of major adverse cardiac events were lower. The most substantial difference was a lower rate of inhospital heart failure in institutions where hs assays were used. This was seen in the overall population and in the ACS cohort. A modest reduction in the delay to angiography without a reduction in the length of stay was observed among patients in hospitals using hs assays. However, the availability of high-sensitivity troponin testing may lead to a larger number of patients at lower risk of ACS, or without an eventual diagnosis of ACS, being admitted. Appropriately designed randomised clinical trials informed by high-quality ACS registries are needed to determine the true incremental value of high-sensitivity troponin testing.
A number of limitations of our study must be considered. Assays may have been misclassified in terms of troponin type (I or T) and the clinical cut-offs reported. Patients were grouped according to the assay used at the hospital they presented to, and we assumed all management was informed by these results. Further, troponin testing results were not available for patients transferred from or to non-participating centres.
In conclusion, the use of highly sensitive troponin assay results in managing patients admitted with suspected ACS is associated with an increase in non-ACS diagnoses with no increase in MI diagnoses. There was a lower rate of major inhospital events, predominantly heart failure, which may be attributable to the larger proportion of non-ACS diagnoses. There is an ongoing need to determine the incremental value of the widespread introduction of highly sensitive troponin assays into routine clinical practice.
1 Discharge diagnoses of 4371 consecutive patients having a first admission with a suspected or confirmed acute coronary syndrome grouped by the type of cardiac troponin assays in use at the hospital to which they presented
|
Final diagnosis |
Sensitive assay |
Highly sensitive assay |
Total |
P |
|||||||||||
|
|
|||||||||||||||
|
STEMI/LBBB |
156 (8.9%) |
262 (10.0%) |
418 (9.6%) |
0.004 |
|||||||||||
|
NSTEMI |
434 (24.8%) |
570 (21.7%) |
1004 (23.0%) |
||||||||||||
|
Unstable angina/likely ischaemic |
395 (22.6%) |
530 (20.2%) |
925 (21.2%) |
||||||||||||
|
Chest pain, unlikely cardiac |
448 (25.6%) |
742 (28.3%) |
1190 (27.2%) |
||||||||||||
|
Other |
314 (18.0%) |
520 (19.8%) |
834 (19.1%) |
||||||||||||
|
Total |
1747 (100%) |
2624 (100%) |
4371 (100%) |
||||||||||||
|
|
|||||||||||||||
|
STEMI = ST-segment elevation myocardial infarction. LBBB = left bundle branch block. NSTEMI = non-ST-segment elevation myocardial infarction. |
|||||||||||||||
2 Resources used for all 4371 patients* having a first admission with a suspected acute coronary syndrome (ACS) and those with (2347) and without (2024) ACS diagnoses according to the type of cardiac troponin assay used
|
ACS diagnosis |
Non-ACS diagnosis |
All patients |
|||||||||||||
|
Investigations and treatments |
Sensitive |
Highly sensitive assay |
P† |
Sensitive |
Highly sensitive assay |
P† |
Sensitive |
Highly sensitive assay |
P† |
||||||
|
|
|||||||||||||||
|
Tests |
|||||||||||||||
|
Exercise stress test |
54 (5.5%) |
116 (8.5%) |
0.005 |
97 (12.7%) |
259 (17.6%) |
< 0.001 |
151 (8.6%) |
375 (14.3%) |
< 0.001 |
||||||
|
Echocardiography |
318 (32.3%) |
544 (39.9%) |
< 0.001 |
146 (19.2%) |
272 (21.6%) |
0.198 |
464 (26.6%) |
816 (31.3%) |
0.001 |
||||||
|
Stress echocardiography |
14 (1.4%) |
25 (1.8%) |
0.439 |
12 (1.6%) |
54 (4.3%) |
0.001 |
26 (1.5%) |
79 (3.0%) |
0.001 |
||||||
|
Stress nuclear scan |
36 (3.7%) |
29 (2.1%) |
0.026 |
38 (5.0%) |
45 (3.6%) |
0.118 |
74 (4.2%) |
74 (2.8%) |
0.011 |
||||||
|
Computed tomography coronary angiogram |
27 (2.7%) |
63 (4.6%) |
0.019 |
23 (3.0%) |
50 (4.0%) |
0.270 |
50 (2.9%) |
113 (4.1%) |
0.014 |
||||||
|
Angiogram |
524 (53.2%) |
802 (58.9%) |
0.006 |
133 (17.5%) |
155 (12.3%) |
0.001 |
657 (37.6%) |
957 (36.5%) |
0.446 |
||||||
|
Procedures |
|||||||||||||||
|
Percutaneous coronary intervention |
279 (28.3%) |
452 (33.2%) |
0.012 |
5 (0.6%) |
3 (0.2%) |
0.146 |
284 (16.3%) |
455 (17.3%) |
0.349 |
||||||
|
Coronary artery bypass graft |
63 (6.4%) |
93 (6.8%) |
0.678 |
1 (0.1%) |
4 (0.3%) |
0.415 |
64 (3.7%) |
97 (3.7%) |
0.954 |
||||||
|
Inhospital therapy |
|||||||||||||||
|
Aspirin |
914 (92.8%) |
1273 (93.5%) |
0.523 |
598 (78.5%) |
858 (68.0%) |
< 0.001 |
1512 (86.6%) |
2131 (81.2%) |
< 0.001 |
||||||
|
Intravenous heparin |
319 (32.4%) |
552 (40.5%) |
< 0.001 |
52 (6.8%) |
100 (7.9%) |
0.363 |
371 (21.2%) |
652 (24.9%) |
0.006 |
||||||
|
Low-molecular-weight heparin |
543 (55.1%) |
685 (50.3%) |
0.021 |
241 (31.6%) |
281 (22.3%) |
< 0.001 |
784 (44.9%) |
966 (36.8%) |
< 0.001 |
||||||
|
GP IIb/IIIa inhibitor |
67 (6.8%) |
123 (9.0%) |
0.051 |
3 (0.4%) |
1 (0.1%) |
0.123 |
70 (4.0%) |
124 (4.7%) |
0.258 |
||||||
|
Discharge treatment‡ |
|||||||||||||||
|
Aspirin |
823 (86.6%) |
1166 (87.6%) |
0.493 |
423 (55.6%) |
637 (50.5%) |
0.028 |
1246 (72.9%) |
1800 (69.8%) |
0.028 |
||||||
|
Other antiplatelet therapy |
585 (61.6%) |
850 (63.9%) |
0.266 |
128 (16.8%) |
213 (16.9%) |
0.963 |
713 (41.7%) |
1061 (41.1%) |
0.706 |
||||||
|
β-blocker |
697 (73.4%) |
928 (69.7%) |
0.058 |
298 (39.1%) |
442 (35.0%) |
0.065 |
995 (58.2%) |
1366 (53%) |
0.001 |
||||||
|
ACEi or AR2B |
674 (71.0%) |
819 (61.5%) |
< 0.001 |
351 (46.1%) |
544 (43.1%) |
0.194 |
1025 (60.0%) |
1362 (52.8%) |
< 0.001 |
||||||
|
Statin |
789 (84.0%) |
1088 (81.7%) |
0.160 |
381 (50.0%) |
604 (47.9%) |
0.351 |
1179 (69.0%) |
1689 (65.5%) |
0.017 |
||||||
|
Inhospital cardiac rehabilitation |
411 (43.3%) |
545 (41.0%) |
0.269 |
103 (13.5%) |
110 (8.7%) |
0.001 |
514 (30.1%) |
654 (25.4%) |
0.001 |
||||||
|
Outpatient cardiac rehabilitation |
464 (48.8%) |
591 (44.4%) |
0.036 |
104 (13.7%) |
109 (8.6%) |
< 0.001 |
568 (33.2%) |
699 (27.1%) |
0.036 |
||||||
|
|
|||||||||||||||
|
ACEi = angiotensin-converting enzyme inhibitor. AR2B = angiotensin-II receptor blocker. GP = glycoprotein. * Sensitive assay, 1747; highly sensitive assay, 2624. † Values derived from univariate (unadjusted) analyses. ‡ Calculated among the 4288 patients (ACS diagnosis, 2281; non-ACS diagnosis, 2007) discharged alive. |
|||||||||||||||
3 Inhospital major adverse cardiac events, including death, myocardial infarction, cardiac arrest* in total, and new-onset heart failure stratified by the availability of highly sensitive and sensitive troponin assays (unadjusted [univariate] analysis)
|
Patients with an ACS diagnosis |
All patients |
||||||||||||||
|
Major adverse cardiac events |
Sensitive assay |
Highly sensitive assay |
P |
Sensitive assay |
Highly sensitive assay |
P |
|||||||||
|
|
|||||||||||||||
|
Died in hospital |
35 (3.6%) |
31 (2.3%) |
0.065 |
38 (2.2%) |
45 (1.7%) |
0.275 |
|||||||||
|
MI/further MI after admission |
31 (3.2%) |
47 (3.5%) |
0.686 |
33 (1.9%) |
52 (2.0%) |
0.828 |
|||||||||
|
Inhospital heart failure |
113 (11.5%) |
122 (9.0%) |
0.045 |
140 (8.0%) |
155 (5.9%) |
0.007 |
|||||||||
|
Total |
164 (16.7%) |
187 (13.7%) |
0.050 |
204 (11.7%) |
238 (9.1%) |
0.005 |
|||||||||
|
|
|||||||||||||||
|
ACS = acute coronary syndrome. MI = myocardial infarction. * Included in the total. |
|||||||||||||||
An economic case for a cardiovascular polypill? A cost analysis of the Kanyini GAP trial
There is increasing global interest in the use of frequently indicated medications in fixed-dose combination for the prevention of cardiovascular disease (CVD).1,2 Evidence of the effectiveness of such polypills as a strategy in improving adherence to recommended treatment and potentially lowering costs is growing.3,4 Although there are combination blood pressure-lowering and cholesterol-lowering medications, a more comprehensive cardiovascular polypill (containing generic aspirin, a lipid-lowering and two blood pressure-lowering agents) is not currently available in Australia. At a feasible cost of less than $1 per day5 compared with a minimum cost in Australia of $1.70 per day for individual generic therapies (http://pbs.gov.au/info/about-the-pbs), prima facie evidence exists for extensive savings from such a strategy in Australia.
The cost-effectiveness of polypill-based strategies compared with individual medications has yet to be tested in real-life settings, although cost-effectiveness has been shown in different patient groups and health care settings using modelled projections.5,6–8 For instance, based on a modelled analysis of high-risk primary care Dutch participants, polypill use after opportunistic screening was cost-effective among people aged over 40 years.8 Similarly, a polypill strategy was found to be cost-effective and potentially cost-saving in older patients after myocardial infarction.7
This analysis is based on the Kanyini Guidelines Adherence with the Polypill (GAP) pragmatic randomised controlled clinical trial and linked health service and medication administrative claims data from Medicare Australia. Kanyini GAP was pragmatic in that it was conducted within the primary care setting, with the study drug dispensed through community pharmacies, to test the effectiveness of a polypill-based strategy in real-world practice.9 In the trial, the polypill improved patients’ adherence to treatment and there was no difference in mean blood pressure and cholesterol levels.3 The results were consistent with a larger sister trial, the UMPIRE (Use of a Multidrug Pill in Reducing Cardiovascular Events) study. Conducted in Europe and India, UMPIRE found that a polypill strategy yielded improvements in self-reported adherence, along with statistically significant but small additional reductions in blood pressure and cholesterol, compared with non-polypill treatment, in the polypill arm.4 A unique design feature of Kanyini GAP was that all medications, including the polypill, were dispensed at an out-of-pocket charge consistent with prevailing Medicare subsidies (around $35 per medication per month for general patients).
Methods
A within-trial cost analysis of the polypill strategy versus usual care was conducted from the Australian health system perspective (ie, government plus patient costs). Kanyini GAP was carried out within Indigenous and non-Indigenous urban, rural, and remote primary care settings across Australia (randomisation from January 2010 to May 2012; median follow-up, 19 months; maximum follow-up, 36 months).3
Data on health service and medication expenditure throughout Kanyini GAP were obtained via individually linked Australian Medicare records for study participants who consented to linkage. Two separate Medicare datasets were analysed: the Medicare Benefits Schedule (MBS), which records government and patient costs of general practitioner and specialist visits and diagnostic tests; and the Pharmaceutical Benefits Scheme (PBS), which records the total government and patient costs of all medications dispensed outside hospital. The PBS data do not include polypill costs, as it is not marketed in Australia. As no difference was found in safety or clinical outcomes between treatment groups in the Kanyini GAP trial, we assumed no differences in hospital-related expenditure.
The primary outcome was mean MBS and PBS expenditure per patient per year. The base year for the analysis was 2012.10 This study was approved by human research ethics committees in all relevant jurisdictions (Sydney South West Area Health Service; Aboriginal Health and Medical Research Council of New South Wales; Cairns Base Hospital; Princess Alexandra Hospital Centres for Health Research; Central Australia; Northern Territory Department of Health and Menzies School of Health Research; Monash University). Each participant provided written informed consent.
Statistical analysis
Multivariable analysis was conducted to accommodate potential differences between treatment groups, given these analyses were restricted to the subset of trial participants consenting to Medicare linkage.11 Non-significant demographic, socioeconomic, health and treatment-related covariates (P > 0.10) were removed via backwards stepwise elimination. To account for the empirical distribution of MBS and PBS cost data,11–14 generalised linear models were used to estimate the primary outcome, and the marginal difference between treatment groups was compared (Wald test). Deb–Manning–Norton programs for Stata 12.1 (StataCorp) were used.12
Results
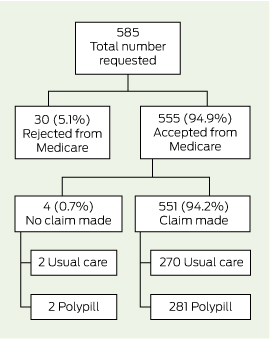
Box 1 and Box 2 detail the flow of patients through this analysis. Consent for linkage with MBS and PBS data was obtained from 93.9% (585/623) and 92.0% (573/623) of trial participants, respectively, and data were provided for 94.9% of participants (MBS, 555/585; PBS, 544/573). With regard to PBS data, 10.8% (62/573) of consenting participants were receiving medications under the special rural and remote access provisions of section 100 of the National Health Act 1953 (Cwlth) and were removed from this analysis, as these data were not captured by Medicare at an individual patient level (Box 2). There was no differential availability of linked data between treatment groups.
The MBS and PBS expenditures by the Australian health system (government plus patient costs) per patient per year, excluding the cost of the polypill, are presented in Box 3. The adjusted analysis predicted a mean cost saving for pharmaceutical expenditure of $989 (95% CI, $648–$1331) per patient per year (P < 0.001) to the health system. No significant differences were found in MBS expenditure.
Discussion
Our study showed that participants receiving polypill-based care had significantly lower pharmaceutical expenditure than usual care, with no difference in health service expenditure. The overall potential savings are dependent on the reimbursement price of the polypill. Under current Australian guidelines, fixed-dose combinations such as the polypill are reimbursed at no greater than the sum of the costs of the generic components,15 which was $1.70 per day at the time the trial was conducted. At this maximum price, and based on an average of 264 days per year on polypill treatment as observed in the treatment arm of the Kanyini GAP study,3 the annual savings to the health system would be $540 (ie, $989 − $1.70 × 264 days) per patient.
The Kanyini GAP trial found that the polypill was safe and effective in improving combination preventive treatment use by patients.3,4 Using primary care expenditure data, our within-trial analysis provides evidence of significant cost savings through the introduction of a CVD polypill, showing its economic dominance over conventional individual therapies. The ACE Prevention project, an Australian economic modelling study,5 also indicated dominance, estimating that a polypill at $200 per person per year was cost saving and resulted in a large population health impact, even when provided to patients at lower risk than those in the Kanyini GAP trial. If we had applied this lower cost in our analysis, we would have estimated annual health system savings of $789 per patient.
Challenges remain before large cost savings can be realised in Australia. First, no polypill has had regulatory approval in Australia to date. While several cardiovascular combinations have been approved, these are simple two-component combinations approved on the basis of straight substitution among patients receiving recommended medications (among whom the benefits are smallest),3,4 and have probably increased costs as a result of not being subject to automatic price reductions.16 Another challenge will be appropriate scale-up while maintaining overall cost savings — large investments will be required in order to bring about practice change for this relatively new way of treating patients.
One limitation with using PBS data is that over-the-counter and very low-cost medications priced at below the government copayment level are not captured. However, this potentially leads to an underestimate of the cost savings as it is likely to include some of the individual cardiovascular medicines in usual care, such as aspirin. Additionally, subsequent reductions in the cost of usual care associated with the expiry of patents for atorvastatin and rosuvastatin since conducting the Kanyini GAP trial may have an impact on the translation of such cost savings into contemporary practice.
This is the first study using individual patient-linked administrative data to evaluate the cost offsets associated with a CVD polypill compared with current practice. Given that over 600 000 Australians at high risk of CVD are currently prescribed antiplatelet, blood pressure and lipid-lowering medication, and a similar number are on partial treatment,17,18 this polypill has the potential to not only help to reduce the large gaps that exist in Australia between recommended and actual treatment for patients with CVD,18 but also to free up considerable amounts of pharmaceutical expenditure.
2 Pharmaceutical Benefits Scheme expenditure
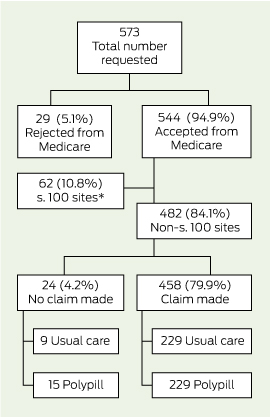
* Patients receiving medications under the special rural and remote access provisions; individual-level data not captured by Medicare.
3 Health system expenditure (government plus patient costs) per patient per year of follow-up*
|
Scheme |
Usual care |
Polypill |
Marginal difference |
||||||||||||
|
|
|||||||||||||||
|
Medicare Benefits Schedule |
|||||||||||||||
|
No. of participants |
270 |
281 |
– |
||||||||||||
|
Unadjusted expenditure, mean (95% CI) |
$1772 ($1581 to $1963) |
$1760 ($1602 to $1917) |
$13 (− $236 to $261)† |
||||||||||||
|
Adjusted‡ expenditure, mean (95% CI) |
$1767 ($1583 to $1951) |
$1770 ($1615 to $1926) |
$40 (− $202 to $281) |
||||||||||||
|
Pharmaceutical Benefits Scheme |
|||||||||||||||
|
No. of participants |
229 |
229 |
– |
||||||||||||
|
Unadjusted expenditure, mean (95% CI) |
$2438 ($2100 to $2775) |
$1443 ($1285 to $1601) |
$995 ($622 to $1368)†¶ |
||||||||||||
|
Adjusted§ expenditure, mean (95% CI) |
$2448 ($2141 to $2754) |
$1446 ($1291 to $1602) |
$989 ($648 to $1331)†¶ |
||||||||||||
|
|
|||||||||||||||
|
* 2012 A$, estimated with generalised linear model (gamma family, log link). † In favour of polypill. ‡ Adjusted for sex, Australian rural and remote area index (http://www.aihw.gov.au/rural-health-rrma-classification), adherence at baseline, history of cardiovascular disease and prior medication use. § Adjusted for sex, Australian rural and remote area index, health care concession status–income interaction, adherence at baseline, history of cardiovascular disease and prior medication use. ¶ P < 0.001. |
|||||||||||||||
Modern challenges in acute coronary syndrome
Despite a growing evidence base, gaps in knowledge and practice leave room for improvement in the treatment of acute coronary syndrome
A few decades ago, there was still controversy about the importance of interruption of blood flow versus myocardial tissue oxygen demand in causing myocardial infarction.1,2 It is now universally accepted that coronary thrombosis at the site of an unstable atherosclerotic plaque is the usual cause of coronary occlusion3 and the cluster of conditions of unstable angina, non-ST-elevation myocardial infarction (NSTEMI) and ST-elevation myocardial infarction (STEMI) comprise the clinical complex now called acute coronary syndrome (ACS).
An important observation from the investigations at the start of the “reperfusion era” was the recognition that STEMI and NSTEMI, while both due to coronary thrombosis, had quite different presentations and natural histories.4 Important differences between the pathophysiology of STEMI and NSTEMI determine the focus of treatment. In STEMI, the complete occlusion of the coronary vessel initiates a cascade of myocardial necrosis, which can be prevented by early reperfusion with percutaneous coronary intervention or fibrinolytic therapy.5 In NSTEMI, the less complete occlusion of the coronary vessel means there is less immediate urgency to salvage myocardium, and the initial focus is on antithrombotic therapy to limit the size and instability of the thrombosis in the coronary artery. In this situation, the size, shape and location of the coronary thrombosis are highly variable. The patient’s clinical course can be unpredictable, and progression to STEMI is a pervading concern. In patients with NSTEMI who are at high risk, an early invasive approach has been shown to be superior to a conservative approach,6 but the optimal timing of this remains controversial.7 These major advances in understanding this symptom complex have driven quantum shifts in management approaches and greatly improved outcomes for patients who have suffered a heart attack. However, it remains a condition which can be unpredictable and, despite the best of modern treatments, can still be lethal. As ACS is a symptom of underlying coronary heart disease, long-term management is often more important than the acute phase. This supplement focuses on the many challenges in managing ACS.
The first two articles in this supplement deal with managing the acute stage of ACS. The many valuable guidelines on this topic,8–12 not reiterated in detail in the supplement, all concur on the basics of modern therapy. The use of potent antithrombotic agents is central to tackling the coronary thrombosis, albeit with an increased risk of bleeding. While controversies continue over the ideal duration of antiplatelet therapy, the evidence to support routine early and post-hospital use of potent antiplatelet agents is overwhelming. Statin therapy is also central to the management of the acute episode and for long-term management, irrespective of the low-density lipoprotein cholesterol level at the time of the episode. The role of β-adrenergic blockers and inhibitors of the renin–angiotensin–aldosterone system remain important, but perhaps better targeted to patients at higher risk. The guidelines, while sometimes exhaustingly complete, do not cover all aspects of management.
In the first article in the supplement, Brieger focuses on the identification of patients with ACS who are at high risk (https://www.mja.com.au/doi/10.5694/mja14.01249). He argues that routine risk stratification as soon as possible after presentation will determine the clinical pathway, and that this practice should be embedded in the hospital system — it is too important to leave to ad-hoc and potentially unreliable clinical judgement. This is a challenging change in approach for the hospital system, but bound to be fruitful in reducing decision time when early revascularisation is needed, and avoiding unnecessary intervention when it is not.
Next, McQuillan and Thompson review the limited evidence to guide management in four important subgroups: female, older, diabetic and Indigenous patients (https://www.mja.com.au/doi/10.5694/mja14.01248). These subgroups have been underrepresented in clinical trials, in contrast with the evidence base that guides the care of most other patients with ACS, which is rich and detailed. There is also evidence that these subgroups are at particular risk, and clinical decisions must often be based on extrapolation from the results of clinical trials without absolute certainty that the evidence is applicable.
The other articles in the supplement deal with the challenges in caring for post-ACS patients at the time of discharge from hospital and handover to the general practitioner. This transition can lead to confusion for the patient and frustration for the GP in dealing with patients returning to their practice with major changes in their management incompletely documented and uncertainty about how best to access the services available to their patients.
Redfern and Briffa use data from three registries to describe common shortfalls in the transition from hospital to primary care (https://www.mja.com.au/doi/10.5694/mja14.01156). The challenges in improving access to effective secondary prevention are concisely summarised, with positive guidance on how to improve secondary prevention in primary care, raising awareness of the need for lifelong secondary prevention, better integration and use of existing services, consideration of the use of registry data in data monitoring and quality assurance, and the potential in embracing new technologies such as automated texting reminders to patients, already outlined in a summit on this topic last year.13
Thompson and colleagues summarise the extensive evidence base for ideal post-hospital therapy (https://www.mja.com.au/doi/10.5694/mja14.01155), focusing on the 50% of patients who do not receive coronary intervention or revascularisation at the time of their acute episode.14 The extensive collaboration on clinical trials and registries that has gone into developing the rich evidence base is a source of pride in modern cardiology, but many gaps in evidence remain.
Thakkar and Chow reassert the truism that drugs do not work in patients who do not take them (https://www.mja.com.au/doi/10.5694/mja14.01157); there is evidence that non-adherence among post-ACS patients is common and associated with adverse outcomes.15 Their review summarises strategies to improve adherence to prescribed medications, and touches on the future possibility of a polypill to include a combination of evidence-based therapies to improve adherence.
Finally, Vickery and Thompson take the GP’s perspective in managing the post-ACS patient and describe eight common challenges that GPs face in this setting (https://www.mja.com.au/doi/10.5694/mja14.01250). The need for courteous, detailed communication between the hospital and primary care is highlighted.
The common theme of each article in this supplement is that progress has been impressive, but much has to be done to continue the improvements in understanding and in translating the knowledge we already have into further improvements in outcomes. The disturbing evidence from recent Australian nationwide surveys that the application of proven evidence-based therapies remains less than optimal16 is a concern and presents a major challenge in the modern management of ACS.
Optimising pharmacotherapy for secondary prevention of non-invasively managed acute coronary syndrome
Despite a trend towards greater use of coronary revascularisation, half of all patients who experienced an acute coronary syndrome (ACS) in Australia in 2012 had their conditions managed non-invasively — that is, they did not receive coronary angiography with subsequent coronary stenting or bypass surgery.1 The evidence base and international guidelines for the management of patients with ACS are extensive,2–4 but some research suggests that patients whose ACS is treated conservatively may not receive the same level of evidence-based care as those whose ACS is managed invasively.5
This article reviews the optimal pharmacological management of non-invasively managed ACS, and briefly reviews the evidence to support the prescription of each class of drug.
Antithrombotic therapy
As coronary thrombosis is the major cause of ACS, antithrombotic treatment regimens are now routine.
Aspirin
Aspirin in a dose of 75–325 mg daily is recommended in all guidelines for all patients after an ACS, regardless of whether revascularisation has occurred.2–4 Its low cost and high effectiveness make it an attractive agent to reduce the risk of recurrence of coronary thrombosis. In post-ACS patients, aspirin has been shown to reduce major vascular events by 25%, with an absolute risk reduction of 35 vascular events per 1000 patients treated over 2 years.6 Prescribing levels well in excess of 95% for post-ACS patients have been reached in Australia.1
A limitation with aspirin therapy, even in low doses, is an increase in the risk of gastrointestinal side effects. A recent meta-analysis calculated that the odds ratio for the risk of major gastrointestinal bleeding in aspirin versus non-aspirin users was 1.55 (95% CI, 1.27–1.90).7 Observational studies suggest that bleeding complications are fewer with lower doses of aspirin,8 but randomised allocation to low-dose (75–100 mg) versus standard-dose (101–325 mg) aspirin in combination with clopidogrel showed no differences in bleeding at 30 days.9 Enteric-coated versions of aspirin may have fewer adverse gastric effects than buffered aspirin, but it remains unclear whether it is the enteric coating or the lower dose that decreases the risk of gastric complications.10 Co-prescribing a proton pump inhibitor (PPI) reduces the risk of gastrointestinal bleeding, but the long-term cost-effectiveness of the combination with aspirin remains doubtful.11
P2Y12 inhibitors and dual antiplatelet therapy
Dual antiplatelet therapy (DAPT; aspirin and a P2Y12 inhibitor drug) is now recommended for conservatively managed post-ACS patients in all guidelines.2–4 The CURE study, conducted nearly 15 years ago, showed a clear role for DAPT in conservatively managed ACS; patients treated with DAPT (clopidogrel and aspirin) had fewer subsequent coronary events than patients treated with aspirin alone.12 At 12 months, the CURE trial’s end point of myocardial infarction or cardiovascular death was reduced by 20% (relative risk reduction, 0.80; 95% CI, 0.72–0.90; P < 0.001). This benefit came with a moderate increase in major bleeding (relative risk, 1.38; P = 0.001). All subsequent guidelines based on the CURE trial data recommend DAPT for conservatively managed ACS.
The ideal duration of DAPT after an ACS episode without percutaneous coronary intervention (PCI) remains unclear. While there are ongoing trials to examine the optimal duration of DAPT in patients treated with PCI,13,14 the relevance of these trial results to conservative management is not clear.
International guidelines recommend the combination of clopidogrel with aspirin for 12 months, as this was the treatment period examined in the CURE trial.2–4 Post-hoc review of the events curves in the CURE study showed that the major benefit of clopidogrel plus aspirin over aspirin alone was in the first 6 weeks after commencement of treatment, and there have been no comparative studies to evaluate shorter or longer periods of therapy. The benefits of longer-term DAPT over aspirin have not been confirmed.15
Concerns about resistance to clopidogrel in some patients have led to extensive research into clopidogrel resistance. “Clopidogrel resistance” is more correctly defined as high on-treatment platelet reactivity and, according to some estimates, up to 30% of patients are non-responders or poor responders to clopidogrel by this criterion.16,17 However, recent studies have shown that dosing based on platelet responsiveness to clopidogrel is unhelpful.18,19
Like aspirin, clopidogrel can increase the risk of gastrointestinal bleeding, and concomitant use of PPIs with clopidogrel has been closely examined, as several observational studies suggested that PPIs may interfere with the action of clopidogrel via competition for the cytochrome P450 pathway in the gut transport of the prodrug.20 However, a well conducted randomised trial of omeprazole showed no clinically significant interaction with clopidogrel,21 and the most recent meta-analyses have shown that an interaction between PPIs and clopidogrel is not significant for most patients. The earlier observations of adverse effects of the combination may have been due to PPI users being older patients, who are at increased risk of adverse cardiovascular events.22
Newer oral agents that inhibit the P2Y12 receptor (ticagrelor, prasugrel) have recently become available. Dosages for the three agents are summarised in Box 1.
Both ticagrelor and prasugrel are more effective in reducing subsequent coronary events, but carry a higher bleeding risk than clopidogrel. Most guidelines recommend that the newer agents are preferred for most ACS (both ST-elevation myocardial infarction and non-ST-elevation myocardial infarction) unless the risk of bleeding is excessive.2–4
Prasugrel was more effective than clopidogrel in the TRITON-TIMI 38 trial in reducing coronary events.23 However, this definitive trial of prasugrel only included patients for whom the coronary anatomy was known, and a coronary angiogram may not be available for patients whose ACS is managed conservatively. Prasugrel was ineffective for conservatively managed ACS.24 Because of bleeding risk, care is required in older patients (> 80 years), and those who weigh under 60 kg or have renal impairment.
Ticagrelor was also shown to be more effective than clopidogrel at preventing stroke, myocardial infarction or death, as demonstrated in the PLATO trial.25 It also has an increase in overall bleeding risk, but as the trial evidence supporting its use did not require prior coronary angiography, it has the advantage as the preferred agent for initial treatment, particularly in the patient whose ACS is likely to be managed conservatively.26
Warfarin and new oral anticoagulants
Post-ACS vitamin K antagonists were evaluated in the 1990s and were shown to achieve a reduction in re-infarction, and are even more effective in reducing the risk of stroke but with an increased risk of bleeding.27 Based on this experience, two of the new oral anticoagulants (NOACs) have been tested in patients who had experienced a coronary event. Rivaroxaban was shown to reduce recurrences, but at an increased risk of bleeding.28 Apixaban did not reduce recurrent ischaemic events and caused increased bleeding.29
Anticoagulants for atrial fibrillation after ACS
Atrial fibrillation (AF) requiring an oral anticoagulant is a common comorbidity in patients with recent ACS being treated with DAPT. Patients with non-invasively managed ACS, AF and a zero CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke, vascular disease, age 65 to 74 years, sex) score can be managed with aspirin or DAPT.30 However, patients with a moderate or high risk of stroke need to be considered for triple antithrombotic therapy (DAPT for their coronary disease and an anticoagulant for their AF), and this carries an increased risk of bleeding. Registry data have quantified this risk, showing that the combination of antiplatelet agents with warfarin increases the risk of bleeding by 1.50 for aspirin and by 1.84 for clopidogrel over warfarin alone.31
There are no trials to guide therapy for patients with non-invasively managed ACS and AF, but a randomised study of participants requiring anticoagulation and antiplatelet therapy after PCI demonstrated that double therapy with clopidogrel and warfarin was associated with significantly less bleeding than triple therapy with aspirin, clopidogrel and warfarin, without any increased risk of thrombotic events.32 To date, there are no data to guide the use of the NOACs with the newer P2Y12 inhibitors, which are becoming standard care for patients after ACS.
Lipid-modulating medications
Statins
Statin therapy is an essential part of the post-ACS regimen. Meta-analyses of trials in patients who have had a coronary event have shown that subsequent coronary events can be reduced by 25%–30%,33 with an absolute reduction of 48 major vascular events per 1000 treated for each 1 mmol/L reduction in low-density lipoprotein (LDL) cholesterol level.34 Commencement of the statin in hospital enhances adherence over subsequent months.35 Lower-potency statins are less effective,36 and a high-dose potent statin is more effective than a moderate-dose less potent statin,37 and equally safe.38 High-dose (80 mg) simvastatin was associated with a higher than acceptable incidence of myopathy in a trial of post-ACS patients and should be avoided.39 While rosuvastatin has been shown to be effective in high-risk non-ACS cohorts, there is no specific trial to support its use after ACS. The target LDL cholesterol level for post-ACS patients is below 1.8 mmol/L.40 It remains unclear whether a patient who achieves a reduction of LDL cholesterol to target levels with 80 mg of atorvastatin should be prescribed a lower-dose statin to reduce side effects.
Many patients experience side effects while taking statins, but analysis of randomised trials has shown that major side effects are equally seen in participants treated with placebo and statins, apart from a small increase in type 2 diabetes.41 While myopathy is uncommon, symptoms of myalgia are common and quite often lead to early cessation of statin therapy.
Non-statin lipid-modulating therapies
Ezetimibe has the potential to lower LDL cholesterol levels, either alone or in conjunction with statins,42 but to date, there are no data to demonstrate any clinical benefit. The outcome of the IMPROVE-IT trial will be awaited with interest to see if lowering LDL cholesterol levels by non-statin therapy is effective.43
PCSK9 inhibitors have been shown to be highly effective in lowering LDL cholesterol levels among patients with hyperlipidaemia resistant to statins, and they have an acceptable safety profile,44,45 but the relevance of this to reducing risk among patients after ACS remains to be established.
Triglyceride-lowering medications
There is no clear-cut benefit for lowering triglyceride levels in patients after ACS. Trials of gemfibrozil46 and bezafibrate47 have not been sufficiently persuasive to establish fibrate therapy in post-ACS patients, and a large trial with fenofibrate did not achieve its primary end point in patients with high-risk type 2 diabetes.48
High-density lipoprotein cholesterol-raising medications
Trials of high-density lipoprotein (HDL) cholesterol-raising drugs have been disappointing. While cholesteryl ester transfer protein inhibitors can raise HDL cholesterol levels, they have not been shown to improve outcomes. A large torcetrapib trial in patients with stable coronary heart disease demonstrated an increased mortality.49 A large dalcetrapib study in patients with ACS showed an effective raising of HDL cholesterol levels, but no effect on outcomes.50 A preliminary study of anacetrapib showed that it could lower LDL cholesterol levels as well as raise HDL cholesterol levels,51 but a large outcomes study with anacetrapib is yet to be reported.
Alternative approaches to raising HDL cholesterol levels have been explored, so far without success. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy raised HDL cholesterol levels, but showed no additional benefit over statin therapy.52 Niacin combined with the anti-flushing agent laropiprant caused an unacceptable increase in risk of myopathy in patients taking simvastatin.53 The challenge in HDL cholesterol management may be to target the functionality of the HDL cholesterol, rather than simply the level.54
Other therapies
Omega 3 fatty acids
Fish oil-derived omega 3 fatty acids have been shown to moderately reduce total and sudden post-ACS deaths, but it is not clear if this is by a triglyceride-lowering effect or other mechanisms.55
β-Adrenergic blockers
β-Blockers are recommended for long-term post-ACS management in most guidelines.2–4 This is sound advice for most patients in the early post-ACS period, but the recommendation for long-term use of β-blockers is based on evidence obtained from clinical trials conducted in the 1980s.56 At that time, the definition of ACS was based on criteria quite different from the modern definition, which is based on subtle changes in troponin.57 In the 1980s, the definition of a myocardial infarction for a patient to be included in a post-myocardial infarction trial required major electrocardiogram changes and a doubling of cardiac enzymes.58 When the post-infarction oral β-blocker trials were conducted,59–61 many modern treatments, such as early intervention with PCI, the near-universal use of statin therapy and the widespread use of DAPT, had not been introduced to cardiology. The relevance of 30-year-old evidence derived from patients with extensive myocardial infarction to the treatment of patients in the modern era is doubtful.62
Recent evidence has shown no benefit of β-blockers on mortality in patients with hypertension63 or stable coronary heart disease,64 casting further doubt on the assumption that routine, long-term use of β-blockers in stable post-ACS patients is essential. In contrast, research among patients with left ventricular (LV) dysfunction or cardiac failure after myocardial infarction shows clear evidence of benefit for β-blockers.65,66
ACE inhibitors and angiotensin receptor blockers
Angiotensin-converting enzyme (ACE) inhibitors have a role in patients with cardiac failure and significant LV dysfunction.67 The use of ACE inhibitors in the absence of post-coronary LV dysfunction has been extensively studied, and meta-analysis of clinical trials in this group of patients shows a statistically significant but modest benefit, with 10 lives saved for 1000 patients treated over 4.4 years.68 A recent large observational registry study did not replicate the benefits of ACE inhibitors seen in clinical trials, and failed to demonstrate any improvement in survival among patients treated with ACE inhibitors.69 Angiotensin receptor blockade as an alternative to ACE inhibition has been trialled in post-ACS patients, but the evidence base for angiotensin receptor blockers (ARBs) is not as extensive as it is for post-infarction ACE inhibitors.70
Aldosterone blockade
Spironolactone and eplerenone have shown benefit in patients with cardiac failure and LV dysfunction.71,72 Eplerenone is approved for authority use on the Australian Pharmaceutical Benefits Scheme for patients with heart failure with an LV ejection fraction of 40% or less occurring within 3 to 14 days after an acute myocardial infarction. If spironolactone or eplerenone are prescribed for the post-ACS patient, meticulous monitoring of potassium levels is required, particularly for patients taking concomitant ACE inhibitors.73
Calcium-channel blockers
Calcium-channel blockers have not been shown to benefit prognosis for the post-infarction patient, and are not recommended as part of routine management. Verapamil and diltiazem are contraindicated in post-infarction patients with LV dysfunction.74,75 Amlodipine has been shown to be safe in the presence of LV dysfunction.76
Antiarrhythmic drugs
Antiarrhythmic drugs are not recommended, as they have not been shown to improve prognosis for patients who have had a myocardial infarction.77
Nitrate therapy
Nitrates are indicated for patients with symptomatic angina, but do not have a role for patients without angina after myocardial infarction.78
Diuretics and digoxin
Diuretics are useful for symptomatic relief of cardiac failure but have not been convincingly shown to improve prognosis for patients after ACS.79 The need for ongoing diuretic therapy should be reviewed at the time of hospital discharge, as unnecessary diuretic therapy can cause hypovolaemia and electrolyte disturbances.
Digoxin does not have a clear role except as an alternative to β-blockers for rate control of AF.80
Conclusions
Recommendations for optimal pharmacotherapy in the post-ACS patient are summarised in Box 2. Most patients will recover without symptoms or LV dysfunction. Patients in this category should be routinely prescribed DAPT and a high intensity statin. The DAPT should be continued for 12 months and the aspirin and statin indefinitely. All patients should be taking a β-blocker when they leave hospital, but the evidence for long-term continuation in the modern era is minimal, and further trials are needed to clarify the ideal duration of β-blocker therapy. While ACE inhibitors or ARBs are recommended in some guidelines, the evidence for their routine use in the patient free of cardiac failure or LV dysfunction is questionable.
Patients who have documented LV dysfunction after their ACS should have the same treatment as above, but in these patients the evidence for β-blockers and ACE inhibitors or ARBs is strong and they should be prescribed. The preferred β-blocker should be one of the agents shown to be effective in clinical trials of cardiac failure or LV dysfunction. There is good evidence that aldosterone antagonists are effective in patients with LV dysfunction.
Symptomatic patients with angina or dyspnoea or cardiac failure should be treated as above and have appropriate treatment for their angina or symptoms of cardiac failure.
1 P2Y12 inhibitor antiplatelet drugs and dosages
|
Drug |
Loading dose |
Maintenance dosage |
|||||||||||||
|
|
|||||||||||||||
|
Clopidogrel |
300 mg or 600 mg |
75 mg daily |
|||||||||||||
|
Prasugrel |
60 mg |
10 mg daily |
|||||||||||||
|
Ticagrelor |
180 mg |
90 mg twice daily |
|||||||||||||
2 Recommended treatment for patients whose acute coronary syndrome has been conservatively managed
|
Clinical scenario |
Drug |
||||||||||||||
|
|
|||||||||||||||
|
Asymptomatic patient without left ventricular (LV) dysfunction |
Aspirin 100–150 mg per day indefinitely P2Y12 inhibitor for 12 months High-dose statin indefinitely β-Blocker (long-term duration uncertain) Consider angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker |
||||||||||||||
|
Asymptomatic patient with LV dysfunction |
As above Substitute a β-blocker shown to be effective in LV dysfunction (bisoprolol, carvedilol, nebivolol) Add ACE inhibitor or angiotensin receptor blocker Consider aldosterone antagonist (spironolactone or eplerenone) |
||||||||||||||
|
Symptomatic patient |
As above For angina symptoms, prescribe standard anti-anginal therapy, consider referral for angiography or revascularisation Prescribe a diuretic for dyspnoea |
||||||||||||||
The transition from hospital to primary care for patients with acute coronary syndrome: insights from registry data
In Australia, acute coronary syndrome (ACS) accounts for about 75 000 hospital separations annually, and in 2010 cost more than $8 billion.1 Those who survive are at high risk of recurrent events; in 2010, more than 25 000 Australian hospital separations were associated with repeat ACS2,3 at a cost of more than $600 million (direct costs only).1 Between 2000–01 and 2008–09, the largest expenditure increase, by health care sector, was for hospital-admitted patient services, where cardiovascular disease expenditure increased by 55%, from $2907 million to $4518 million.4 A recent report projected that by 2020 there will be around 102 363 separations associated with ACS in Australia, and about half of these will be due to repeat events.1 These statistics highlight the growing importance of secondary prevention as more people survive initial events. Further, it underscores the need for a health system that has an inbuilt process for commencing prevention during acute admissions, and the need to ensure an effective transition from hospital to primary care.
Favourable modification of coronary risk factors is responsible for at least a 50% reduction in mortality from cardiovascular disease.5,6 Further, participation in secondary prevention programs leads to improved clinical, behavioural and health service outcomes, including fewer hospital readmissions, better adherence to pharmacotherapy, enhanced functional status, improved risk profile, less depression, and better quality of life.7–9 However, only a minority participate,10 systematic follow-up is fragmented,11 and questions remain about how well the health system facilitates transition from hospital to primary care. Overall, with ACS dominating expenditure and gaps in secondary prevention widely documented, addressing the delivery of care at the point of hospital discharge is a priority.
Modern cardiology has seen significant advancements in diagnosis, revascularisation, pharmacotherapy and overall more successful treatment of acute illness.12 This ultimately means that more people are surviving their initial ACS event and are having shorter hospital stays, which has resulted in more people returning to the community and resuming their everyday lives.13 However, one-quarter of survivors will be readmitted to hospital within 1 year of the index event, and a significant number of readmissions will end in death.2,3 Consequently, the demand for effective, continuing post-hospital preventive care is intensifying; the foundations of this are built during the acute episode.14
The purpose of this article is to provide insights from registries, and their implications for secondary prevention in Australia.
Gaps in secondary prevention: data from Australian ACS registries
Three large-scale Australian ACS registries — namely, SNAPSHOT ACS, the Cooperative National Registry of Acute Coronary care, Guideline Adherence and Clinical Events (CONCORDANCE) and the Acute Coronary Syndrome Prospective Audit (ACACIA) registry — have provided contemporary data about secondary prevention and resource gaps.
The SNAPSHOT ACS audit provides recent data pertaining to pre- and inhospital ACS care in Australia and New Zealand.12 The audit involved the collection of detailed information about 4398 consecutive patients admitted to 483 public and private hospitals across the two countries over 2 weeks in May 2012.12 The ACACIA registry enrolled 3402 ACS patients from 39 hospitals across Australia (25% rural, 75% metropolitan).3 CONCORDANCE is an ongoing (prospective) clinical initiative that provides continuous real-time reporting on the clinical characteristics, management and outcomes of hospitalised ACS patients to clinicians, hospital administrators, sponsors, interested stakeholders and government.15 CONCORDANCE currently includes about 5200 patients from more than 40 hospitals.
As a group, the Australian ACS registries provide detailed and contemporary information about inhospital care. All three registries show suboptimal rates of pharmacotherapy and cardiac rehabilitation referral. The proportion of patients prescribed at least four of the five indicated pharmacotherapies at discharge was 68% in CONCORDANCE16 and 65% in SNAPSHOT ACS.12 In terms of cardiac rehabilitation, 58% were referred in CONCORDANCE17 and 46% in SNAPSHOT ACS.12
A recent article resulting from SNAPSHOT ACS reported that only 27% (628/2299) of Australian and New Zealand patients admitted to hospital for ACS received a combination of guideline-recommended medications, referral to rehabilitation and basic lifestyle advice before hospital discharge.11 The authors suggested that a greater focus on inhospital delivery of preventive care is needed to provide the essential foundation for lifelong secondary prevention.11
However, it should also be noted that registries have limitations, such as the reliance on inhospital documentation, and they may not be able to determine individual contraindications.
The challenges in implementation of secondary prevention
There are well known limitations in the implementation of secondary prevention after ACS. Despite proven effectiveness and clear recommendations in best-practice guidelines,18 there is poor use of effective medications, cardiac rehabilitation and adherence to lifestyle recommendations.19 In the recent AusHEART survey of more than 5000 Australian general practice patients, 1548 had clinically expressed cardiovascular disease, and only half of these were following recommended treatments.20 Valid national data on participation in cardiac rehabilitation and exercise therapy are not available, but estimates from local and international reports indicate that less than 30% of eligible patients participate in such programs.10,21 Compliance with lifestyle change is no better. It was recently reported that among 18 809 patients from 41 countries who had experienced ACS, only 30% of patients adhered to diet and exercise recommendations, and about two-thirds of smokers had quit smoking 6 months after their event.22
Overall, it is difficult for a coherent strategy to emerge when the volume of evidence describing and reporting disparate models of delivery continues to expand.13 In reality, about 70% of Australian secondary prevention programs continue to follow the traditional cardiac rehabilitation model of structured and group-based exercise with education sessions.19 These programs are associated with well documented barriers, including the need for transport, poor health provider support, limited time frames and minimal individualisation. Thus, policymakers, health professionals and researchers are confronted by the need for increased services to improve access and equity, but often with significant challenges coupled with finite and declining resources.13
Opportunities for improved access to and uptake of secondary prevention
A recent blueprint for reform summarises the outcomes of a national summit that aimed to improve implementation of secondary prevention in Australia.23 The report identifies stakeholder consensus for an approach where each patient’s acute episode of care, particularly at discharge and follow-up, is patient-centred. The report also highlights the current challenges associated with the existence of a divide between hospital and general practice care. That divide is apparent in terms of a definition of prevention and rehabilitation, patient communication, service provision, funding and data collection. The summit report also summarises opportunities for improved implementation of secondary prevention.23 Although not exhaustive, the published opportunities present practical suggestions that cover a range of issues, including public health support, better coordination and use of existing strategies, workforce, quality assurance and technology, as follows:
- Increased delivery of comprehensive secondary prevention in primary care
- Provision of connected care through a case-management approach, improved communication, and greater provider education relating to secondary prevention, behaviour change techniques and self-management strategies.
- This model of care should be coupled with specific incentive programs similar to those already available for diabetes and asthma management.
- Possible development of a role for cardiac care coordinators, who would ideally be recognised by Medicare. These coordinators could collaborate with the person with ACS and other members of the care team to achieve mutually agreed clinical targets, good health and wellbeing.
- Increased focus on and awareness of the need for lifelong secondary prevention
- Potential for widespread media and public awareness campaigns to raise the profile and understanding of ACS as a chronic condition requiring lifelong management.
- Requires linking with and engagement of state and federal governments, Medicare Locals, consumers and private health funds to facilitate sustainability.
- Better integration and use of existing services
- Better use of existing initiatives, such as cardiac rehabilitation, chronic disease management plans, private health insurance programs, and other initiatives including the Home Medicines Review, Heart Foundation programs (eg, Heartmoves) and Quitline.
- Better use and awareness of these existing programs may require development of a comprehensive inventory, database or website, and could be the domain of a national preventive agency, ideally in collaboration with state governments, Medicare Locals and non-government organisations, but could otherwise be housed by an established non-government organisation such as the National Heart Foundation.
- Data monitoring and quality assurance
- Identification of performance measures to enable cross-national comparison is needed for post-hospital care. This should incorporate measures of service delivery as well as health outcomes, including hospital readmissions and coronary heart disease deaths.
- This could occur via an online registry and/or electronic medical records and data linkage.
- Embracing new technologies
- New technological developments have seen a rapid rise in devices and trials aimed at managing cardiovascular disease risk factors, medication adherence and providing coordinated care.
- Ongoing development and testing of technological advances may facilitate greater access to secondary prevention.
- Examples of e-health approaches include the use of text messaging, telephone-delivered care, development of websites and smartphone apps and remote monitoring and remote delivery of programs.
The increasing role of primary care in ACS management
ACS requires lifelong management, and primary care is ideally positioned to provide this care to patients.23 There is a need to go beyond giving patients a discharge summary and advising them to make an appointment. In one study, about 20% of patients did not have a discharge summary forwarded to their general practitioner, and 68% of GPs rated the information in the summaries they received as “very good” to “excellent”.24
In Australia, there has been a substantial shift in the payment system for GPs towards incentives that encourage evidence-based care of patients with chronic diseases in line with a disease management framework that emphasises systematic, coordinated care and self-management. The Australian Government’s commitment to a National Primary Health Care Strategic Framework provides an opportunity to establish primary care systems and funding models to enable people who are at high risk of a cardiovascular event to be identified early for preventive care.25 The National Heart Foundation maintains that a well developed primary care framework for secondary prevention will increase referral and access rates to secondary prevention services, enhance continuity of care and improve coordination of services between hospitals and the community.26
Conclusion
Despite guideline advocacy, uptake of proven secondary prevention strategies for heart disease is suboptimal. Australian registries provide contemporary data that reinforce the evidence–practice gaps in secondary prevention. Trial and cohort data highlight the need to commence prevention early if we are to narrow the divide between hospital and the community, thereby achieving better individual, provider and system-level outcomes. Patients often leave hospital without systematic follow-up and with an unclear picture of how and if they will be managed and supported on the next phase of their chronic disease journey. Potential opportunities to bridge the divide include development of an incentive scheme in primary care, development of a cardiac care coordinator role to work in concert with treating doctors and patients, better use of existing services, effective data monitoring and embracing new technologies.
Management of acute coronary syndrome in special subgroups: female, older, diabetic and Indigenous patients
While guidelines for the management of acute coronary syndrome (ACS) for the average patient are well defined, their application to special groups is less clear. Some groups have been underrepresented or formally excluded from the clinical trials that constitute the evidence base that guides therapy, and some have special needs that have not been studied in clinical trials. As a result, these subgroups present clinical challenges for which there is weaker evidence to define appropriate management.
This article will assess the special needs of women, older people, patients with diabetes, and Aboriginal and Torres Strait Islander Australians.
Women
Cardiovascular disease remains the leading cause of death in women, and far exceeds deaths from cancer at all ages. Despite the popular perception that heart attack is far more common in men than women, this is true only in younger age groups. Across the full spectrum of age, women account for a large proportion of patients with ACS, comprising up to 45% of patients in some series1 and 40% in a comprehensive snapshot of admissions to coronary care units in Australia and New Zealand in 2012.2
There is considerable evidence that the pattern of coronary disease and ACS is different in men and women.3,4 Women tend to have less obstructive coronary artery disease and a higher prevalence of microvascular coronary dysfunction,5 leading to the suggestion that the term ischaemic heart disease may be more appropriate for women than coronary artery disease.6
While most women presenting with an ACS will experience typical anginal symptoms, women are less likely to report chest pain or discomfort compared with men. They are more likely to report associated symptoms including dyspnoea, nausea and fatigue.7 These differences may be partly explained by the higher average age at ACS presentation among women, with older patients of both sexes less likely to report chest pain.7 There is a higher prevalence of stress or takotsubo cardiomyopathy among women, usually in the absence of obstructive coronary disease.8 There are also some differences in the reference range and pattern of troponin elevation between the sexes.9 Overall, however, the diagnostic and prognostic performance of troponins is similar in men and women.10 These differences complicate the diagnosis of ACS in women, and may contribute to delays or deficiency in the delivery of guideline-indicated therapies.
The outcomes after an ACS are worse for women than men.11 The most likely explanation is that women have their heart attacks about a decade later in age than men. After detailed multivariate analysis of age and other prognostic factors, being female is not an independent risk factor for non-ST-elevation ACS (NSTEACS), but it is for ST-elevation myocardial infarction (STEMI).12 This disparity in STEMI is particularly evident in younger women (< 55 years old), who have a higher risk of death and recurrent events compared with similarly aged men.13,14
Concerns have been expressed that women may not receive the same benefit from invasive management as men experiencing an NSTEACS. Individually, the trials comparing conservative with invasive management were each underpowered to demonstrate a benefit in women. Reports from meta-analyses of the trials give conflicting results. Trials to 2008 showed a clear benefit in women at higher risk,15 but a more recent meta-analysis including the data from the OASIS 5 trial failed to demonstrate any benefit for women from an early invasive approach.16 It is clear that women receive invasive management less frequently than men, probably because of higher rates of comorbidity rather than a treatment bias,17 but do not fare any worse solely because of a lower rate of intervention.18
Women are less likely to receive guideline-indicated pharmacotherapies after ACS. The clinical trials for guiding pharmacotherapy after an ACS episode have tended to include far fewer women than men. As a result, the evidence base to guide therapy in women is less robust than for men.19 Despite this, there is no clear evidence that the usual post-ACS medications are less efficacious in women than in men. The available evidence shows equivalent efficacy for the post-ACS use of aspirin,20 clopidogrel21 and ticagrelor,22 as well as the short- and long-term use of β-blockers,23 statin therapy24 and angiotensin-converting enzyme inhibitors25 after myocardial infarction. Due to generally lower body weight and estimated glomerular filtration rates, especially among older women, caution is required to avoid excess dosing of antithrombotic therapy. Meta-analysis of the available clinical trials of hormone replacement therapy has shown no benefit, but no adverse effect of hormone replacement therapy after ACS.26
Older people
Older people comprise a large proportion of patients with ACS. In the United States-wide CRUSADE study, patients aged 75 years and older comprised 35% of all ACS patients.27 The risk of a poor outcome after ACS rises with age, and each of the widely used scoring systems shows a major influence of age. In the Thrombolysis in Myocardial Infarction (TIMI) score for STEMI patients, an age of 75 years or older had an adjusted odds ratio of 2.7 for predicting 30-day mortality.28 In the Global Registry of Acute Coronary Events (GRACE) score for patients with NSTEACS, each decade of increasing age was associated with an adjusted odds ratio of 1.7 for predicting 30-day mortality, with those aged 75 years having an eightfold higher risk of death at 30 days compared with a 40-year-old.29,30 In 8557 patients from Australia and New Zealand enrolled in the LIPID Study, the rate of death or myocardial infarction over a mean of 6 years was 19.6% in patients aged 70 years and older compared with 11.2% in patients younger than 60 years, with a hazard ratio of 1.89 (95% CI, 1.60–2.23).31
Because of their higher absolute risk of adverse cardiovascular events after ACS, patients aged 75 years and older have potentially more to gain from appropriate therapies if they offer similar risk reduction across all ages. Despite this, there is a well recognised paradox that older patients who are at highest risk often receive less active treatment. The tolerability, safety and adverse event profile of these therapies must also be carefully considered for older patients. Clinical trials have enrolled lower proportions of older patients, with lower rates of comorbidities especially renal impairment, stroke and congestive heart failure, than are present in community series.27 The proportion of patients presenting with atypical symptoms or non-diagnostic electrocardiogram changes increases with age, which may result in delays in the diagnosis of ACS and institution of appropriate therapies.32 Patients’ preferences and their perception of risks associated with invasive management must also be taken into account.
Although concerns regarding bleeding risks and vascular complications including stroke may explain lower rates of invasive strategies among older patients with ACS, similar patterns are observed for pharmacotherapy. Although each of the evidence-based post-ACS treatments has been shown to be effective for older patients,33 there may be a reluctance to treat this group. Some of the reluctance for intervention and pharmacotherapy in older patients no doubt reflects sensible clinical judgement, as there may be increased risks with some medications with age.
The relative benefit of aspirin does not appear to be affected by age. Compared with younger patients, people aged 65 years and older had a greater absolute reduction in vascular end points and lower death rate with aspirin use after ACS.34 For inhibitors of adenosine diphosphate-dependent platelet aggregation, when added to aspirin in the management of ACS, there is divergence in the risk–benefit profile for older patients. Clopidogrel offers similar absolute risk but lower relative risk reduction when given to patients older than 65 years compared with younger patients, although in both groups clopidogrel is more effective than placebo.35–37 Patients aged 75 years and older and those with a body weight below 60 kg demonstrated no net clinical benefit from prasugrel in the TRITON–TIMI 38 study of ACS patients with scheduled percutaneous coronary intervention (PCI).38 Those with prior stroke or transient ischaemic attack, which are more prevalent among older patients, had net harm with prasugrel. Ticagrelor was superior to clopidogrel in the overall PLATO trial, and has been shown to be of similar efficacy in reducing subsequent cardiovascular events among patients aged 75 years and older compared with younger patients.39 Bleeding rates were similar to those with clopidogrel in patients 75 years and older and not significantly different to rates in those younger than 75 years included in PLATO. Dyspnoea and ventricular pauses were more common with ticagrelor, but at similar rates regardless of age.
Statins for secondary prevention of cardiovascular events have consistently demonstrated benefit compared with placebo, with most trials finding greater absolute risk reduction among patients aged 65 years and older compared with those younger than 65 years.40 These findings have been extended in the PROSPER41 and Heart Protection42 studies, which included larger numbers of patients aged 75–80 years, with similar event reduction in each of the age groups. Meta-analysis of statin trials examining the benefit of more versus less aggressive lowering of low-density lipoprotein cholesterol levels for secondary prevention of cardiovascular events has shown similar efficacy in those aged older than 65 years compared with patients aged 65 years and under.43 For ACS trials, efficacy has been shown with high-dose atorvastatin compared with lower-dose atorvastatin44 or pravastatin,45 without evidence of differential treatment benefits due to age. Statin therapy may have more side effects in older patients, particularly in higher doses, and closer surveillance is warranted.46
Older patients also have a higher likelihood of renal dysfunction that is not necessarily identified from serum creatinine levels,47 and some studies estimate that significant renal dysfunction is present in up to 35% of people over the age of 70 years.48 The likelihood of worsening renal dysfunction from use of contrast medium can be a factor in decision making for older patients who are being considered for invasive therapy.49 Many cardiovascular drugs are cleared by renal mechanisms, and dosing considerations should be observed.50 Renal dysfunction can also be a significant factor in increasing the risk of bleeding with anticoagulants, particularly with low-molecular-weight heparins, which are cleared via the renal route.51,52 Registry data show that excessive doses of antithrombotic agents are frequently given to older patients.50
Routine invasive therapy, which has a well recognised benefit in reducing myocardial infarction and recurrent angina compared with a selective invasive strategy,53 can have an even greater benefit in older patients at high risk. The TACTICS-TIMI 18 trial compared the impact of invasive and conservative strategies in younger (aged 65–75 years) and older (aged over 75 years) patients. The early invasive strategy conferred an absolute reduction in end points of 10.8% in younger patients, and 21.6% in older patients (P = 0.016).54 Despite this evidence of greater benefit, international55 and Australian56,57 studies have shown that older patients at higher risk routinely receive less invasive management than younger patients.
Explanations for this paradox in the delivery of invasive therapy vary, but there are valid clinician concerns about comorbidities, interaction with concomitant medications and increased fragility, which are more likely explanations than an “ageist” bias. Concerns about a higher risk of bleeding in older patients may restrict the use of invasive therapies, as bleeding has been shown to be an independent predictor of a poor outcome after an ACS event.58
People with diabetes
Diabetes is an important risk factor for cardiovascular disease, with a threefold increased risk of ACS, and an increased risk of post-infarct heart failure, recurrent ischaemia and death.59 In Australia, 25% of patients who present with ACS have diabetes.2 Compared with patients without diabetes, patients whose ACS occurs in the setting of diabetes tend to have more extensive coronary artery disease60 and angioscopic evidence of more unstable disease with ulcerated plaques and intracoronary thrombi.61 There is evidence of disordered cardiac function in diabetes which may be independent of myocardial ischaemia,62 and includes a high prevalence of diastolic ventricular dysfunction.63
Patients with poor glycaemic control during their ACS have a worse outcome. Even modest elevations of plasma glucose levels in patients without known diabetes, previously referred to as “stress hyperglycaemia”, is related to increased mortality.64 While intensive medical therapy to achieve strict glycaemic control in patients with diabetes has been shown to reduce the risk of microvascular complications and some cardiovascular disease events in long-term follow-up,65,66 there have been inconsistent results in the context of ACS. While small early open-label clinical studies appeared to have favourable results,67 subsequent larger blinded randomised controlled trials failed to confirm any clear benefit of insulin- or sulfonylurea-based therapy to tightly control glucose levels.68 The use of fixed-rate glucose, insulin and potassium (GIK) infusions has been studied in several trials, including the CREATE-ECLA study in patients with STEMI,69 with prespecified analyses combined with the GIK component of the OASIS-6 trial.70 These trials included more than 20 000 patients, 5000 of whom had diabetes. There was no beneficial effect of GIK infusion on outcomes, with possible harm resulting from excess fluid load, although the early excess in mortality became neutral at 30 days. When prehospital GIK therapy was administered to patients with suspected ACS, the IMMEDIATE trial demonstrated no initial benefit71 or improvement in overall outcomes at 1 year.72
The role of intensive insulin therapy in ACS patients has been further informed by studies of strict glycaemic control in critically ill patients. The Australian NICE-SUGAR trial in multicentre intensive care settings demonstrated increased mortality with intensive glycaemic control and emphasised the serious adverse consequences of hypoglycaemia in these patients.73 There was a lower mortality rate with a blood glucose target of below 10.0 mmol/L (< 180 mg/dL) than with a target of 4.4–6.1 mmol/L (81–108 mg/dL).73 Current guidelines have recognised the differences between long-term benefits of intensive glycaemic control in younger patients with more recent diabetes onset, and the lack of benefit or potential harm among the critically ill or older patients with longstanding diabetes at the time of ACS.74 The focus should be on active management of hyperglycaemia, with blood glucose levels below 11.0 mmol/L while avoiding hypoglycaemia. Dose-adjusted insulin infusions with regular monitoring of blood glucose levels should be considered, but routine administration of insulin and glucose with or without potassium is not recommended unless there is a clinical indication.
Trials of most guideline-indicated pharmacotherapy for patients with ACS and diabetes have demonstrated similar or greater risk reduction than for patients without diabetes. Dual antiplatelet therapy with aspirin and clopidogrel has demonstrated similar benefits for patients with diabetes compared with overall trial participants, although people with diabetes face a higher residual risk of recurrent events, possibly reflecting more persistent platelet activation. Prasugrel and ticagrelor offer more rapid and complete platelet inhibition and superior net clinical benefit to clopidogrel in studies of patients with diabetes and ACS, with similar overall bleeding rates in patients with diabetes.75,76 Inhibitors of platelet glycoprotein IIb/IIIa receptors (including abciximab, eptifibatide and tirofiban) may have a role in high-risk ACS patients, including people with diabetes who are undergoing PCI.77
Statins have been shown to offer consistent benefit for secondary prevention of cardiovascular events among patients with diabetes, compared with placebo. The Cholesterol Treatment Trialists’ analysis included more than 1000 participants with type 1 diabetes, mainly middle-aged people with prior cardiovascular disease, and found a similar risk reduction to that seen with type 2 diabetes.78 Meta-analysis of trials examining more intensive versus moderate statin therapy have confirmed similar benefits for participants without diabetes.79 For patients with diabetes and ACS, high-dose atorvastatin was superior to pravastatin, with a greater absolute risk reduction than was observed in patients without diabetes. It was noted that only 38% of participants with diabetes achieved the intended goals of a low-density lipoprotein cholesterol level below 1.81 mmol/L (< 70 mg/dL) and a high-sensitivity C-reactive protein level below 2 mg/L.80 Dyslipidaemia is common among people with diabetes, and has been recognised as an independent predictor of adverse outcomes. Trials to date have demonstrated the primary role of statins, but there is some evidence of benefit for fibrates in patients with diabetes and dyslipidaemia for long-term secondary prevention.81,82
For ACS, patients with diabetes compared with the overall population obtain similar or greater reduction in mortality and recurrent myocardial infarction with an early invasive strategy and revascularisation when possible.83 The choice of revascularisation approach is complicated by superior results with coronary artery bypass graft (CABG) surgery compared with PCI in patients with diabetes and multivessel disease, even when new-generation drug-eluting stents are used.84,85 These trials, however, did not typically include patients with ACS. The recommendations of the American College of Cardiology Foundation/American Heart Association guidelines are that for patients with diabetes, single-vessel disease in the setting of NSTEACS86 or acute coronary occlusion of the infarct-related vessel in STEMI87 should be managed with PCI, but CABG is preferred for the management of multivessel disease.
Aboriginal and Torres Strait Islander people
Coronary heart disease is twice as prevalent in the Australian Indigenous population as the non-Indigenous population.88 This population has a high prevalence of cardiovascular risk factors89 and established atherosclerosis.90 A detailed 2006 report by the Australian Institute of Health and Welfare showed that Indigenous Australians had three times the rate of major coronary events and more than twice the inhospital coronary heart disease death rate of other Australians.91 The high incidence affects remote-dwelling92 and urban-dwelling Aboriginal Australians.93,94 The disparities are even more striking in younger age groups. In a Western Australian study, the incidence of coronary events in the 25–29-years age group was 27 times (men) and 35 times (women) higher than in non-Aboriginal participants of the same age, decreasing to two to three times the non-Indigenous rates at 70–74 years.95 A higher frequency of comorbid conditions, primarily diabetes, adversely affects short- as well as long-term survival.96
There are complex sociocultural barriers to delivery of optimal care for Indigenous patients with ACS.97 Lower rates of treatment have been reported. The Australian Institute of Health and Welfare study of 200688 reported a 40% lower rate of being investigated by angiography, a 40% lower rate of coronary angioplasty or stent procedures and a 20% lower rate of CABG surgery. These apparently lower rates of coronary revascularisation in Aboriginal patients disappeared when patients were matched for age, sex and comorbidity. After this adjustment, the rates and type of intervention (including PCI and CABG surgery) during an ACS event were identical.98 Similarly, the adjusted rates of evidence-based prescribing for post-hospital secondary prevention were similar in Aboriginal and non-Aboriginal patients.99
Conclusions
Some subgroups of patients experiencing ACS require special attention in assessment and management. While women, older people, people with diabetes and Indigenous patients may be underrepresented in clinical trials, there is evidence that most guideline-based therapies offer similar benefits in these subgroups. Barriers to routine prescription of effective post-ACS therapies in these patients need to be overcome.

 more_vert
more_vert