During the past four decades several groups in the USA and Europe have developed and clinically implemented different cardiac prostheses or total artificial hearts (TAH)—the Cooley/Liotta TAH, Jarvik7 TAH, Bücherl Heart TAH, AbioCor TAH, and CardioWest TAH (TAH-t), which is currently in use. The aim of TAHs is to mimic native heart anatomy and function, fitting into the pericardial cavity and providing pulsatile unidirectional blood flow from the atria to the great arteries in patients with end-stage biventricular heart failure.
Preference: Cardiology and Cardiac Surgery
99
Cardiac troponin testing for diagnosis of acute coronary syndromes in primary care
Acute coronary syndromes (ACS) are a leading cause of illness and death in Australia. Around 75 000 Australians are hospitalised for ACS each year, with $8 billion spent annually on related inpatient care.1 While mortality caused by ACS is declining because of better control of coronary risk factors and the introduction of new treatments,2 at least 10 000 Australians still die each year as the result of ACS.1
The spectrum of ACS includes unstable angina, where atherosclerotic plaque rupture leads to arterial occlusion and myocardial ischaemia, and myocardial infarction, where ischaemia progresses to myocardial cell necrosis. Further classification into ST elevation myocardial infarction (STEMI) and non-ST elevation myocardial infarction (NSTEMI) is based on electrocardiographic (ECG) findings. Overall, the rate of inhospital major adverse cardiac events caused by ACS (death, cardiac arrest, recurrent myocardial infarction, worsening heart failure, major bleeding or stroke) approaches 30% for STEMI and 20% for NSTEMI.3 Patients with unstable angina are also at increased risk of death and subsequent myocardial infarction, even in the absence of myonecrosis.4
Diagnosing ACS is challenging in primary care as well as in the tertiary setting; 15% of patients who experience an ACS initially contact their general practitioner.5 The diagnosis of ACS in primary care is not always straightforward; signs and symptoms alone are neither sensitive nor specific in the prehospital population,6 and the validity of clinical prediction rules for ACS in primary care populations is limited.7
Given these limitations, there are potential benefits to using cardiac biomarkers in primary care. Cardiac troponin (cTn) is the main biomarker in patients who present with possible ACS. A change in cTn levels signifies myocardial necrosis with high sensitivity and specificity, and allows differentiation of myocardial infarction from unstable angina.8 Examples of the benefits of cTn as a biomarker in primary care include the diagnosis of myocardial infarction where it was not suspected initially because of atypical presenting features; the exclusion of myocardial infarction in low-risk patients; and the conservation of resources by avoiding hospital referral.9
However, there are pitfalls and practical considerations associated with cTn as a biomarker in primary care. Compared with those presenting directly to hospital, patients with ACS who first consult a community physician have longer prehospital delay10 and decreased survival.11 Several authors have expressed concern that GP cTn requests contributes to these outcomes,12,13 and there is also evidence of over-interpretation of positive results12 and over-reliance on negative results.14 There can also be problems with follow-up if the test results are notified after normal practice hours.
In this study, we examined a population of patients with possible symptoms of ACS who underwent GP-initiated cTn testing. We compared the incidence of ACS and associated adverse outcomes with those in patients who had presented to hospital for cTn testing. We also explored GPs’ knowledge of the limitations of the usefulness of cTn testing, and the influence of cTn test results on their diagnosis and hospital referral practices.
Methods
Study design, setting and participants
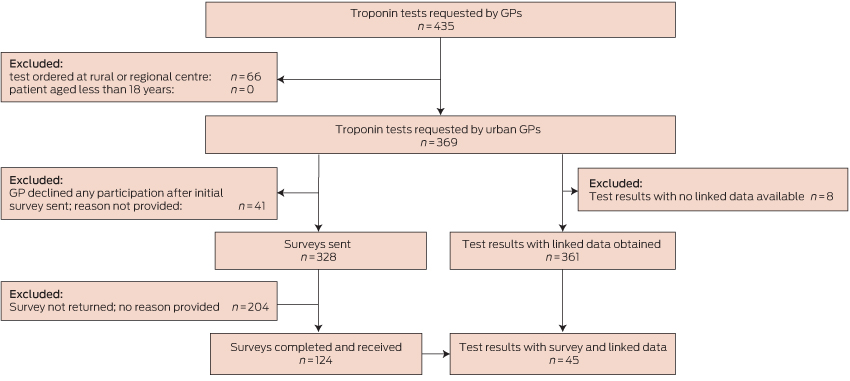
This study employed a prospective cohort design. We recruited patients who had cTn blood tests ordered by a GP in a non-hospital setting, and who had their sample collected at the community collection centres of two of five pathology laboratories in urban Perth, Western Australia. The period of recruitment was 24 September 2009 – 3 September 2010. Patients with samples collected at rural and regional centres were excluded because it was considered likely that GPs in those areas would employ cTn testing differently to urban GPs. Additional exclusion criteria were: patients less than 18 years of age, cTn tests ordered by non-GP doctors, tests ordered for emergency department (ED) patients, and tests ordered by GPs who declined to participate in the study (Box 1).
Data sources and measurement
GP cohort: laboratory data
A research assistant at each laboratory obtained consecutive cTn test results requested by GPs, using the practice address to establish GP status.
GP cohort: survey data
Laboratory research assistants approached requesting GPs for de-identified details about the clinical scenario leading to the cTn test request and the clinical course after notification of the result. GPs were contacted within 1 week of testing, with telephone follow-up to non-responders 1 week after the initial contact. Information was collected on a one-page survey sent and returned by fax, with an information sheet and consent form concurrently sent to the doctor. Risk stratification was undertaken using elements of the National Heart Foundation/Cardiac Society of Australia and New Zealand (NHF/CSANZ) criteria that could be readily assessed during a general practice consultation.15
GP cohort: linked data
Linked data were obtained from the Department of Health Western Australia Data Linkage System (WADLS) for all patients for a minimum 12-month period after the date of their test, irrespective of whether their GP had responded to the survey. The final cTn test included in our study was performed in September 2010, and follow-up continued until October 2011. Outcomes were defined according to standardised definitions recommended for Australasian ACS research.16 Specific diagnosis and procedure codes were selected from the International Classification of Diseases, 10th revision, Australian modification (ICD-10 AM)17 and the Australian Classification of Health Interventions.18 Linkage and extraction were performed in November 2013 to compensate for delay in updating of Department of Health records. Records were excluded from analysis if no principal diagnosis was stated, or if the presenting symptom or principal diagnosis was insufficiently specific to allow classification. Duplicate records with more than one hospital admission for the same patient on the same day were treated as one admission for the purposes of statistical analysis.
ED cohort
Clinical presentations and outcomes in the GP survey cohort were compared with an ED cohort using the Multiple Infarct Markers in Chest Pain (MIMIC) study dataset.19 This prospective cohort study was conducted between September 2008 and June 2009 in two tertiary and three general hospitals in urban Perth. The urban catchment areas of the hospitals were similar to those of the collection centres in the GP survey cohort. Participants were a representative sample of patients undergoing evaluation for possible ACS with serial cTn testing. Patients were excluded if they were less than 18 years of age or pregnant, and where ECG criteria had indicated urgent reperfusion therapy.
Statistical methods
All statistical analyses were performed with SAS version 9.4. Differences between group characteristics were assessed by two-sample t test for continuous variables and by χ2 and Fisher exact tests where appropriate, based on expected frequencies for dichotomous variables. Statistical significance was defined as P < 0.05.
Ethics approval
Ethics approval to conduct the survey was obtained from Human Research Ethics Committees of the University of Western Australia (RA/4/1/2275; 13 July 2009), St John of God Hospital (370; 7 May 2009), the Department of Health Western Australia (2013.04.02; 9 April 2013) and the South Metropolitan Health Services Board (08.136; 28 August 2014). The medical directors of the participating laboratories gave consent for the provision of laboratory data, and ethics approval was obtained from their institutional ethics committees (details available on request).
Results
Participants
Box 1 depicts participant flow through the study. There were no significant differences between included and excluded patients with respect to age or sex (each P > 0.10).
Descriptive data
Box 2 presents the characteristics of the 124 patients in the GP cohort for whom survey data were available. The most common presentation was pain typical of cardiac ischaemia (55.6%).
Data on coronary risk factors were available for 104 GP cohort patients. Six patients (5.7%) were at high risk of ACS according to the NHF/CSANZ risk stratification framework,15 with a combination of typical symptoms and diabetes. A further 65 patients (62.5%) were at intermediate risk of ACS, including 40 (38.5%) over 65 years of age, 16 (15.4%) with various combinations of hyperlipidaemia, a family history of coronary heart disease (CHD), smoking history and hypertension, and nine patients (8.7%) with diabetes and atypical symptoms of ACS.
The median time from specimen collection to sample registration at the processing laboratory was 31 minutes (range, 0 min–1465 min). This interval depended on the location of the collection centre; centres co-located with laboratories had the shortest intervals. Overall, the median time between specimen collection and availability of the test result was 128 minutes (range, 23min –1466 min).
Before receiving the test results, most GPs (80/124, 64.5%) rated the likelihood of ACS in their patient as low (less than 5%). This proportion increased after the results were received (to 110/124, 88.7%). A significant proportion of GPs (34/124, 27.5%) changed their assessment of the likelihood of ACS after a negative test result (χ2 test, P < 0.001).
Most GPs (85/124, 68.5%) intended to manage the patient themselves before receiving the test result, rather than referring them to a hospital or cardiology. This increased to 95/124 GPs (76.6%) after the results were known. Despite the test result having a significant effect on the estimated likelihood of ACS, it did not significantly influence the intended management of the patient (χ2 test, P = 0.23).
The prevalence of smoking (P = 0.01), hypertension (P = 0.02), dyslipidaemia (P = 0.03) and a personal or family history of CHD (P < 0.001) were all significantly greater in the GP survey group than in the MIMIC dataset cohort (Box 2).
Outcome data
GP cohort: linked data
Linked data were available for 361 tests performed for 355 patients; data for eight patients could not be linked because of insufficient identifying information.
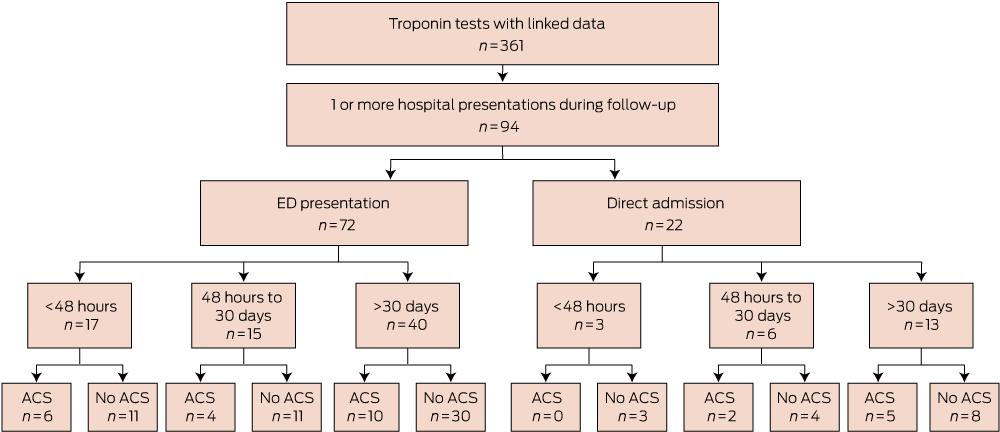
There were 176 presentations to hospital with a cardiovascular symptom or diagnosis during follow-up, whether by presentation to an ED (112 presentations) or by direct admission (64 presentations). Of the 112 presentations to an ED in the GP cohort, 87 were assigned a triage category of 1 or 2, indicating that they required medical review immediately or within 10 minutes.
In total, 94 of 355 of the GP cohort (26.5%) presented at least once to a hospital during follow-up with cardiovascular diagnoses (Box 3 and Box 4). Twenty-one of these 94 patients (22.3%) presented to a hospital within 48 hours of testing. The median time from testing to first presentation was 33 days (range, 0 days–551 days).
Within 48 hours of testing, six of the GP cohort (1.7%) had been diagnosed with an ACS; the median time from specimen collection to hospital presentation for these patients was 382 minutes (range, 80 min–1312 min). Box 5 lists the components of delay for this group.
Within 30 days of cTn testing, 13 of 355 patients in the GP cohort (3.7%) had at least one ACS. Complications included one death from a cardiovascular cause (occurring outside of hospital within 1 week of the test, in a 55-year-old patient), one cardiac arrest in a patient with known CHD, and one episode of cardiogenic shock. During the follow-up period, 27 of 355 patients (7.6%) had at least one ACS. The median time to presentation with the first ACS was 42 days (range, 0 days–498 days).
GP cohort: survey and linked data
For the 124 patients with both linked and survey data, there were 45 presentations to hospital, including 18 ACSs in 11 patients. Six occurred within 1 month of the cTn test, and in each case symptoms had commenced at least 48 hours before the test.
ED cohort
Three hundred and sixty-eight patients of the 1758 in the MIMIC dataset (20.9%) received a discharge diagnosis of ACS, significantly more than the 13 patients (3.7%) with an ACS in the GP cohort (P < 0.001). Most (242/368, 65.8%) were at high risk of ACS according to NHF/CSANZ criteria, with 114 (31.0%) at intermediate risk and 12 (3.3%) at low risk.
Discussion
This study found that most patients who underwent GP-initiated cTn testing had presented typical symptoms of coronary ischaemia and had clinical risk factors indicating intermediate or high risk of an ACS and associated adverse outcomes.15 While most results of GP-initiated cTn tests were negative, such a result did not rule out the possibility of an ACS, as 3.7% of patients (13/355) were admitted to hospital with an ACS within 30 days of receiving a negative result. This approximates the 30-day event rate for patients presenting to an ED and classified as being of intermediate risk.15 In an ED, such patients would not be considered safe for discharge home until further investigations and monitoring had determined a lower risk level.15 The patients in our study, in contrast, would have been largely unmonitored in the community for some hours while awaiting their test results, as well as during the days following a negative result.
The finding that patients undergoing GP-initiated cTn testing were not low-risk was unexpected, and there may have been other factors not detected by the survey that reduced the risk status of patients. Obtaining comprehensive data on individual risk factors may have helped to resolve this question, including ECGs, quantitative blood pressure and lipid profiles, and the results of earlier invasive investigations for CHD. This information would also allow application of additional cardiovascular risk scoring tools and improve the generalisability of our study, although this would risk patient identification and reduced participation because of the longer survey duration.
Turnaround times in this study indicated that there was a substantial delay between presentation to a GP and cTn results becoming available. Particularly concerning was the median delay of more than 5 hours in those patients who were subsequently confirmed to have an ACS and who had presented within 48 hours of symptom onset, when the risk of complications is greatest.8 While the Royal Australian College of General Practitioners Standards20 require evidence of systems that ensure timely response to pathology results, there is evidence from the Threats to Australian Patient Safety (TAPS) study21 and elsewhere9 which suggests that this does not always occur.
GPs may not fully understand the limitations of cTn testing, as 23.4% of tests were ordered within 12 hours of symptom onset (Box 2), at which point the test may be insufficiently sensitive. While all major guidelines groups recommend serial testing to exclude ACS in this context,4,8,22 no serial testing was performed by GPs in our study.
In many cases, the test result did not alter patient management. Some tests were clearly ordered in response to a patient request, and one GP commented that “the test was mainly arranged to satisfy the patient that this was unlikely cardiac”. It is worth noting that a negative cTn test in this context may not have resolved patient anxiety, as many patients presented to a hospital within hours of receiving a negative test.
GPs are in a difficult situation. The consequences of missing an ACS diagnosis can be grave, yet there are no reliable clinical predictors of ACS, and primary care investigations have their limitations. At the same time, GPs have an important role as gatekeepers of the health system.23 Failure to accept any uncertainty may lead to unnecessary investigations and referrals, themselves potential causes of patient harm and unnecessary system costs. However, based on the results of our study, we concur with previous authors in this journal7,9 who have suggested that GPs should maintain a high threshold for requesting cTn testing and refer patients promptly to hospital for assessment when clinical features suggest a diagnosis of ACS. Possible ACS is one setting in which GPs can justifiably advise patients to present to a hospital, rather than undertaking investigations in primary care.
Box 2 –
Characteristics of the patients in the GP and ED (MIMIC dataset)19 cohorts
|
Characteristic |
GP cohort (n = 124) |
ED cohort (n = 1758) |
P |
||||||||||||
|
|
|||||||||||||||
|
Median age, years (interquartile range) |
61 (45–73) |
62 (50–74) |
0.38 |
||||||||||||
|
Sex (male) |
55 (44.4%) |
984 (56.0%) |
< 0.01 |
||||||||||||
|
Cardiac troponin (cTn) test result∗ |
|||||||||||||||
|
Positive |
2 (1.6%) |
168 (10.7%) |
< 0.01 |
||||||||||||
|
Negative |
122 (98.4%) |
1408 (89.4%) |
< 0.01 |
||||||||||||
|
Risk factors† |
|||||||||||||||
|
Smoker‡ |
15 (12.1%) |
425 (24.2%) |
0.01 |
||||||||||||
|
Hypertension |
51 (41.1%) |
923 (52.5%) |
0.02 |
||||||||||||
|
Dyslipidaemia |
47 (37.9%) |
842 (47.9%) |
0.03 |
||||||||||||
|
Diabetes |
15 (12.1%) |
327 (18.6%) |
0.07 |
||||||||||||
|
Past history of CHD or equivalent |
8 (6.5%) |
621 (35.3%) |
< 0.01 |
||||||||||||
|
Family history of CHD§ |
24 (19.4%) |
879 (50.0%) |
< 0.01 |
||||||||||||
|
Presenting symptoms |
|||||||||||||||
|
Typical pain |
69 (55.6%) |
||||||||||||||
|
Atypical pain |
27 (21.8%) |
||||||||||||||
|
Non-pain symptoms |
24 (19.4%) |
||||||||||||||
|
No symptoms¶ |
3 (2.4%) |
||||||||||||||
|
Not recorded |
1 (0.8%) |
||||||||||||||
|
Symptom duration at time of presentation to GP |
|||||||||||||||
|
Less than 12 h |
29 (23.4%) |
||||||||||||||
|
12 h–48 h |
33 (26.6%) |
||||||||||||||
|
More than 48 h |
57 (46.0%) |
||||||||||||||
|
Not recorded |
5 (4.0%) |
||||||||||||||
|
Estimated glomerular filtration rate (mL/min/1.73 m2) |
|||||||||||||||
|
Less than 30 |
3 (2.4%) |
||||||||||||||
|
30–60 |
19 (15.3%) |
||||||||||||||
|
More than 60 |
63 (50.8%) |
||||||||||||||
|
Not recorded |
39 (31.5%) |
||||||||||||||
|
|
|||||||||||||||
|
ED = emergency department; CHD = coronary heart disease. ∗ED cohort: 1576 patients with 8 h–12 h cTn level data. †GP cohort: 104 patients with complete risk factor data. ‡Current smoker or previous smoker of > 10 pack-years. §First or second degree relative < 60 years of age. ¶Reasons for cTn: to investigate elevated creatine kinase levels; for monitoring of cardiac status while on statin; psychiatry patient at risk of cardiac complications of treatment. |
|||||||||||||||
Box 4 –
Details of 94 hospital presentations by members of the GP cohort during follow-up
|
|
|||||||||||||||
|
Acute coronary syndrome |
27 |
||||||||||||||
|
Death outside hospital due to cardiovascular cause (1); cardiac arrest (1); cardiogenic shock (1); ST elevation myocardial infarction (1); non-ST elevation myocardial infarction (5); acute myocardial infarction (9); unstable angina (9)∗ |
|||||||||||||||
|
Coronary heart disease, not otherwise specified |
8 |
||||||||||||||
|
Cardiomyopathy |
1 |
||||||||||||||
|
Heart failure |
5 |
||||||||||||||
|
Arrhythmia |
6 |
||||||||||||||
|
Supraventricular tachycardia (1); ventricular tachycardia (1); atrial fibrillation (1); atrioventricular block, 2nd degree (1); bradycardia (1); cardiac arrhythmia, other (1) |
|||||||||||||||
|
Other cardiovascular diagnosis |
47 |
||||||||||||||
|
Aortic valve stenosis (1); hypertensive (6); chest pain, anterior chest wall (3); chest pain on breathing (26); chest pain unspecified (4); syncope (1); dizziness (3); palpitations (2); dyspnoea (1) |
|||||||||||||||
|
|
|||||||||||||||
|
∗No hospital admission data were collected for four patients with unstable angina. |
|||||||||||||||
Box 5 –
Delay components in six patients presenting with acute coronary syndrome within 48 hours of a cardiac troponin (cTn) test
|
Age (years) |
Collection to registration (min) |
Registration to result (min) |
Collection to result (min) |
Result |
eGFR |
Result to hospital presentation (min) |
Diagnosis |
||||||||
|
|
|||||||||||||||
|
55 |
41 |
164 |
205 |
1.16 |
NR |
398 |
AMI |
||||||||
|
67 |
6 |
107 |
113 |
< 0.10 |
70 |
80 |
UA |
||||||||
|
69 |
6 |
149 |
155 |
1.16 |
66 |
251 |
AMI |
||||||||
|
70 |
163 |
86 |
249 |
3.66 |
49 |
357 |
NSTEMI |
||||||||
|
85 |
12 |
101 |
113 |
< 0.10 |
43 |
1108 |
UA |
||||||||
|
87 |
1188 |
66 |
1254 |
0.14 |
66 |
1312 |
NSTEMI |
||||||||
|
|
|||||||||||||||
|
eGFR = estimated glomerular filtration rate (mL/min/1.73 m2); NR = not recorded; AMI = acute myocardial infarction; UA = unstable angina; NSTEMI = non-ST elevation myocardial infarction. |
|||||||||||||||
News briefs
New collaboration for NHMRC and Americans
The National Health and Medical Research Council (NHMRC) reports that it has opened a joint funding round with their American counterparts, the United States National Institutes of Health (NIH) as part of the United States Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative. Under this collaboration, the NHMRC will provide funding to support Australian researchers to participate in The BRAIN Initiative, which was announced by President Obama in 2013. “It is hoped that the research conducted through The BRAIN Initiative will lead to more effective treatments and methods of prevention for brain conditions such as dementia, autism, epilepsy, depression and Parkinson’s disease”, the NHMRC statement read. The NHMRC CEO Professor Anne Kelso said: “Both the NIH and the NHMRC believe that the ambitious goals of The BRAIN Initiative can best be attained by collaborating across both disciplinary and geographic boundaries. Over the past four decades Australian researchers have collaborated more with researchers in the US than in any other country.”
Missing microbes may point to asthma risk
NPR reports that a new study published in Science Translational Medicine shows that the composition of microbes living in babies’ guts may play a role in whether the children develop asthma later on. “The researchers sampled the microbes living in the digestive tracts of 319 babies, and followed up on the children to see if there was a relationship between their microbes and their risk for the breathing disorder … the researchers report that those who had low levels of four bacteria were more likely to develop asthma by the time they were 3 years old. To further test their theory, the researchers gave laboratory mice bred to have a condition resembling asthma in humans the four missing microbes. The intervention reduced the signs of levels of inflammation in their lungs, which is a risk factor for developing asthma.” The bacteria are from four genuses: Lachnospira, Veillonella, Faecalibacterium and Rothia.
“Predatory” journals publish 400k papers in 2014
Retraction Watch reports that a new analysis by BioMed Central shows that in 2014 so-called “predatory” open-access (OA) journals published around 420 000 papers, up from 53 000 in 2010, appearing in 8000 active journals. “Predatory” OA journals allegedly sidestep publishing standards in order to make money from article processing charges (APC). “Lately, most predatory journals are published by smaller publishers, which maintain between 10 and 99 titles”, Retraction Watch wrote. “The average APC was US$178, and most were published within 2–3 months after being submitted. Predatory journals have made the news — this year, The International Archives of Medicine was delisted from the Directory of Open Access Journals after it accepted a bogus study claiming chocolate had health benefits within 24 hours. In 2013, the same author behind that chocolate study, John Bohannon, tricked more than half of a sample of 300 OA journals to accept fake papers submitted under a fake name and institution. Last year, the Ottawa Citizen tricked a cardiology journal into publishing a paper with a garbled blend of fake cardiology, Latin grammar and missing graphs, for the price of US$1200.”
Cut and paste “tattoo” monitors health 24/7
An inexpensive wearable patch that continuously monitors vital signs for health and performance tracking has been developed by engineers in Texas, Futurity and Engadget report. The “tattoo” is manufactured via a repeatable “cut-and-paste” method that cuts production time from several days to only 20 minutes. “After producing the cut-and-pasted patches, the researchers tested them and discovered they picked up body signals that were stronger than those taken by existing medical devices, including an ECG/EKG, a tool used to assess the electrical and muscular function of the heart. The patch also conforms almost perfectly to the skin, minimising motion-induced false signals or errors. The wearable patches are so sensitive they may be worn to more easily maneuver a prosthetic hand or limb using muscle signals.”
Social network for doctors and their case photos
A new photo-sharing social network called Figure 1 is gaining popularity with doctors, nurses, paramedics and other medical workers, Wired reports. “Figure 1 is educational, engaging, and privacy-obsessed.” Anyone can join, but only health care professionals can comment on photos, which, says Wired, “keeps the discourse focused and professional”. The app is also heavily moderated. An image will be blocked if it doesn’t pose some kind of medical question. The app is very careful about patient privacy. “Every time anyone uploads an image, the first thing they do is fill out a consent form. Figure 1 has an algorithm that automatically obscures faces, and tools that let the user erase any pixels containing names, dates, or any other identifying details.” Figure 1 also strips away all the metadata before the picture gets uploaded. No data collection, over 500 000 users and so far, no ads. “Some of the pictures are straight up medical oddities. But just as often, users post because they are stumped and looking for a 2nd, 3rd, 4th, nth opinion.” The app is available from the iTunes App Store, Google Play and figure1.com.
Catecholamine-induced cardiomyopathy resulting from life-threatening funnel-web spider envenoming
We present two cases of cardiomyopathy in life-threatening funnel-web spider envenoming. A 33-year-old man bitten by a male Sydney funnel-web spider developed autonomic and neuromuscular excess, pulmonary oedema, hypotension and cardiogenic shock. He was treated with antivenom, dobutamine, noradrenaline and high-dose insulin, and recovered over 4 days. Echocardiograms showed severe systolic dysfunction, and high catecholamine concentrations were measured in his blood. A 13-year-old girl developed cardiogenic shock after a funnel-web spider bite, confirmed on echocardiogram treated with antivenom and dobutamine. Funnel-web spider envenoming appears to cause catecholamine-induced cardiomyopathy and cardiogenic pulmonary oedema resulting from catecholamine excess. Antivenom did not reverse the cardiomyopathy.
Clinical records
Patient 1
A 33-year-old man was bitten by a male Sydney funnel-web spider (Box 1) in Newcastle and developed perioral paraesthesia, widespread fasciculations, diaphoresis, hypersalivation and difficulty breathing within 5 minutes. He immediately called an ambulance and was treated with 3 mg atropine on the way to hospital. He arrived in the emergency department (40 minutes after being bitten) with shortness of breath (respiratory rate, 28 breaths per minute), hypoxia, mild hypertension (blood pressure, 143/104 mmHg) and tachycardia (150 beats per minute) with widespread ST changes on electrocardiogram (ECG) (Appendix 1, A). He received two vials of antivenom immediately and was given morphine and midazolam for severe anxiety and apparent pain. A portable chest x-ray showed unilateral patchy consolidation on the right side. He continued to deteriorate with worsening hypoxia, generalised fasciculations and persistent tachycardia, and became delirious, probably secondary to atropine toxicity. He was given two further vials of antivenom after 1 hour and non-invasive ventilation was commenced.
An echocardiogram showed severe systolic dysfunction (ejection fraction [EF], 20%) consistent with his progressing clinical appearance of cardiogenic shock with hypotension, cold peripheries, tachycardia, pulmonary oedema (coughing up frothy fluid) and respiratory acidosis (pH, 7.09 [reference interval (RI), 7.35–7.45]; Pco2, 82.8 mmHg [RI, 35–48 mmHg]; HCO3, 23.7 mmol/L [RI, 25–31 mmol/L]; lactate, 2.0 mmol/L [RI, 0.5–1.6 mmol/L]). A repeat chest x-ray showed infiltrates in both lung fields. He was intubated, infusions of dobutamine (up to 12 µg/kg/min) and noradrenaline (up to 0.16 µg/kg/min) were commenced and he was transferred to intensive care (Box 2; Appendix 2). Adequate cardiac output could not be achieved with these inotropes (cardiac index, 1.3–1.6 L/min/m2 [RI, 2.6–4.2 L/min/m2] measured on a Vigileo monitor [Edwards Lifesciences]), ventricular bigeminy was occurring, and the patient had persistent hypotension, so high-dose insulin therapy (5–10 U/kg/h) was initiated about 6 hours after the bite. Extracorporeal membrane oxygenation was considered but required transfer to Sydney. He finally stabilised about 16 hours after the bite, with a cardiac index of 3.6 and resolution of haemodynamic instability. The inotropes were gradually weaned over 48 hours and a repeat echocardiogram 48 hours after the bite showed an EF of 45%. He was extubated and required intravenous fentanyl for severe chest, shoulder girdle and arm pain. He was transferred to the ward on Day 5 and discharged at 10 days with an EF of 60% on echocardiogram. A progress echocardiogram 6 weeks after the bite was normal, as was an ECG at 12 weeks (Appendix 1, B).
His troponin levels peaked at 5681 ng/L (RI, < 26 ng/L), creatine kinase levels peaked at 2947 U/L (RI, 1–370 U/L), and he had leukocytosis (white cell count, 23.6 × 109; RI, 4–11 × 109). The concentration of funnel-web spider venom was measured by enzyme-specific immunoassay and was 8 ng/mL on admission (before receiving antivenom). The patient had high levels of adrenaline (1.5 nmol/L; RI, < 1.0 nmol/L), noradrenaline (57 nmol/L; RI, < 3.5 nmol/L), dopamine (3482 pmol/L; RI, < 250 pmol/L) and catecholamine metabolites on admission. These decreased over 2–6 hours, except for noradrenaline levels, which increased with noradrenaline therapy.
Patient 2
A 13-year-old girl was bitten on the left ring finger by a funnel-web spider on the south coast of New South Wales at about 2:40 am. There was immediate intense pain, followed by incontinence of urine. She was driven to hospital and had profuse sweating and vomiting. On arrival 35 minutes after the bite, she appeared pale and sweaty with generalised piloerection. Her blood pressure was 155/124 mmHg, heart rate was 84 beats per minute, and oxygen saturation was 98% on room air. She developed shortness of breath and lip and facial swelling, which prompted treatment with adrenaline (400 µg), promethazine, ranitidine, hydrocortisone and dexamethasone. Further deterioration over the next 2 hours occurred with development of pulmonary oedema and hypotension. Her ECG showed ST depression in the inferior and anterior leads (Appendix 1, C). She was given two vials of funnel-web spider antivenom 3 hours after the bite, was intubated for hypoxia and commenced on an adrenaline infusion (0.1 µg/kg/min). She was given a further two vials of antivenom because she had not improved and was transferred to a tertiary paediatric centre. An echocardiogram showed systolic dysfunction (EF, 40%) and mild mitral valve regurgitation. Adrenaline was changed to noradrenaline (0.12 µg/kg/min), and dobutamine (5 µg/kg/min) was commenced for ongoing hypotension and worsening lactic acidosis (lactate, 8.4 mmol/L; pH, 7.20; HCO3, 13 mmol/L; base excess, -13 mEq/L [RI, -2 to + 2]).
Her troponin levels peaked at 646 ng/L, creatine kinase levels peaked at 3365 U/L and she had early leukocytosis (white cell count, 21 × 109). She stabilised over 24 hours, was weaned off inotropes over 48 hours and was extubated on Day 2. Funnel-web spider venom concentrations were 3.2 ng/mL on admission. Repeat echocardiogram on Day 6 before discharge from hospital showed improved ventricular function with an EF of 50%.
Discussion
Funnel-web spiders are the most venomous spiders worldwide1 and envenoming remains a potentially life-threatening condition, for which early administration of antivenom is essential.2 Bites by funnel-web spiders are rare and envenoming only occurs in a small proportion of cases. Envenoming causes autonomic (cholinergic and adrenergic) and neuromuscular excitation, with massive catecholamine release. This results in a characteristic clinical presentation with autonomic excess, muscle fasciculations and paraesthesia. Severe envenoming is associated with pulmonary oedema in about half of cases, which has been assumed to be due to direct venom effects or non-cardiogenic mechanisms.2–4 Antivenom was introduced in 1981 and there have been no deaths since that time.2,5
The autonomic excess and catecholamine release resulting from funnel-web spider envenoming is similar to that seen with severe scorpion envenoming in many parts of the world.6,7 Midventricular stress cardiomyopathy with Carukia barnesi jellyfish envenoming has also been reported,8 consistent with its association with endogenous catecholamine release.9 This would suggest that the acute pulmonary oedema is cardiogenic in origin and results from a catecholamine-induced myocarditis like Takotsubo cardiomyopathy.
A previous report of funnel-web spider envenoming described a myocardial injury similar to these two cases, although the role of catecholamines was not recognised.10 In our first, more severe case, there were increased levels of catecholamines within hours of the bite. The echocardiogram showed severe systolic dysfunction characteristic of a stress-induced acute myocardial injury. Antivenom did not appear to improve the patient’s clinical condition, despite early administration of two vials 1 hour after the bite and a repeat dose of two vials about 2 hours after the bite. This is consistent with the fact that massive catecholamine release had already occurred, resulting in myocardial injury, which is not amenable to antivenom treatment. This is similar to scorpion envenoming, where early antivenom appears to prevent severe envenoming but not improve cardiogenic pulmonary oedema once it develops.6 Patient 2 had similar echocardiogram results confirming a catecholamine- or stress-induced cardiomyopathy that also did not respond to antivenom.
Takotsubo cardiomyopathy cannot be diagnosed unless a patient has normal coronary arteries. Although not clinically indicated in our patients, it is highly unlikely that either patient would have coronary artery lesions, being young and healthy. The pathophysiology of Takotsubo cardiomyopathy remains unclear, but excessive sympathetic nerve activity and massive release of catecholamines are believed to play a role.11 Scorpion cardiomyopathy is thought to share characteristics of Takotsubo cardiomyopathy, where myocardial injury is postulated to be caused by microvascular spasm and microvascular vasomotor dysfunction.7 Our cases suggest that funnel-web spider envenoming causes a catecholamine-induced cardiomyopathy, like Takotsubo cardiomyopathy and very similar to that observed in scorpion envenoming.
The treatment of cardiogenic shock in Patient 1 was controversial because most clinicians would avoid the use of catecholamine inotropes.12 However, dobutamine is used routinely in treating scorpion envenoming and we therefore administered it for both patients.6,7 Patient 1 remained hypotensive despite receiving dobutamine, so high-dose insulin was added because it is a non-catecholaminergic inotrope that acts by improving cardiac metabolism and oxygen use,13 and has previously been used in treating scorpion envenoming.6 High-dose insulin euglycaemia treatment is also used for cardiogenic shock from ß-blocker and calcium antagonist overdoses, with doses ranging from 1 U/kg/h up to 10 U/kg/h;13,14 this was the rationale for the doses used for Patient 1. Other inotropes such as phosphodiesterase inhibitors could potentially be used, along with cardiac assist devices or extracorporeal membrane oxygenation. The acute pulmonary oedema in Patient 1 was treated initially with non-invasive and then mechanical ventilation, and pharmacological interventions were focused on treating cardiogenic shock.
Our report of two patients developing catecholamine-induced cardiomyopathy and cardiogenic shock from confirmed funnel-web spider bites provides evidence that the mechanism of pulmonary oedema resulting from funnel-web spider envenoming is cardiogenic and similar to that resulting from scorpion envenoming.
[Articles] Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double-blind, placebo-controlled trial
Methylprednisolone did not have a significant effect on mortality or major morbidity after cardiac surgery with cardiopulmonary bypass. The SIRS trial does not support the routine use of methylprednisolone for patients undergoing cardiopulmonary bypass.
[Editorial] Coca-Cola’s funding of health research and partnerships
In a bid to increase transparency, Coca-Cola has disclosed spending US$118·6 million in the past 5 years on scientific research and health and wellbeing partnerships. In a list of organisations funded by Coca-Cola, published on Sept 22, they reveal several influential medical organisations that have received funding, including the American Cancer Society, which received roughly $2 million, the American College of Cardiology, which received roughly $3·1 million, and the Academy of Nutrition and Dietetics, as detailed in an article published on Sept 22 in The New York Times.
A change in legislation could increase heart transplant numbers
More hearts could become available for transplant if key terms were altered in transplant legislation, experts say.
According to an Ethics and Law article published in the Medical Journal of Australia, the current legislation has resulted in only 39 hearts being procured from 189 donors (21%) during the first 6 months of 2015.
Associate Professor James Tibballs, Deputy Director of the Intensive Care Unit at Royal Children’s Hospital in Melbourne, and Dr Neera Bhatia, Lecturer at the Deakin University School of Law in Melbourne believe this could change.
They write in their article that the law defines death as either “irreversible cessation of all functions of the brain” (brain death) or “irreversible cessation of circulation of blood in the body” (circulatory death).
Related: Are potential organ donors missed on general wards? A 6-month audit of hospital deaths
Tibballs and Bhatia write that the problem is the legislation doesn’t define irreversible or how to determine irreversibility.
“The fact that a transplanted heart can function and sustain life in a recipient must mean that the circulation of the donor had never ceased irreversibly and therefore that the donor of the heart was never dead until his or her heart was removed.
“The question is thus posed — how is it possible to procure the heart of a donor under the premise of circulatory death and yet expect it to sustain life in a recipient?”
They say this legal grey area means a transplant surgeon could possibly be committing a criminal act by transplanting after circulatory death.
They have provided a possible alternative, being: “Retain the present definition of brain death as irreversible cessation of all function of the brain, but to omit the requirement for irreversibility in the definition of circulatory death and to redefine it as cessation of circulatory function with cessation of higher brain function”, they suggested.
They feel that with such an alteration could increase heart transplant numbers and improve outcomes for the organ donation program.
“Otherwise, Australia’s improving organ donor program is at risk of adverse publicity and damage if doctors, hospitals and our organ procurement agencies are perceived as procuring organs from patients not legally dead,” they write.
A podcast with Associate Professor Tibballs and Dr Bhatia is available at www.mja.com.au/multimedia/podcasts and iTunes. Also available as a video at www.mja.com.au/multimedia
Related: MJA article rejected by transplant experts
Latest news:
- Medibank’s too private actions rile doctors and patients
- Lyme disease – no evidence it is endemic in Australia
- Lost productive life years caused by chronic conditions in Australians aged 45–64 years, 2010–2030
The Cardiac Genetics Clinic: a model for multidisciplinary genomic medicine
Inherited heart disease can be well managed by preventive strategies if detected early. Building on an expanding body of literature on the contribution of hereditary heart disease to sudden cardiac death (SCD)1–3 and the well-validated principles of predictive gene testing in other single-gene disorders, the Cardiac Genetics Clinic (CGC) was formally established at the Royal Melbourne Hospital in 2007. Published data have supported the benefit of clinical screening in such clinics.4 However, detection of a causative mutation, where possible, also allows identification of individuals who are currently clinically unaffected.
The CGC embodies a multidisciplinary model for translating research into international best-practice care.5 This model exemplifies the translation of genetics to genomics in practice, and also aims to educate and inform individuals, allowing them to assume responsibility for their own ongoing care and health.
The CGC is a joint undertaking by the clinical genetics and cardiology units at the Royal Melbourne Hospital. It is managed by a cardiac trained nurse who performs telephone intake on all referrals, and as well as coordinating screening tests and collating relevant clinical information on individuals and their family members before the clinical appointment.6 On average, patients wait 4 to 5 months between referral and appointment. Cardiologists, clinical geneticists and genetic counsellors, as well as fellows and trainees in each field, attend the clinic. Patients may attend individually or with family members; families of deceased patients generally attend as a family unit. Clinics are preceded by a multidisciplinary planning meeting, allowing discussion and decision-making as a group — a particularly important step in making equitable and consistent decisions regarding access to clinic-funded genetic testing and evidence-based medical advice.
The referral base includes general practitioners, physicians and cardiologists; self-referrals are also possible. Relatives of individuals who have died unexpectedly or with autopsy findings suggestive of a genetic cardiac condition are referred by the forensic pathologists at the Victorian Institute of Forensic Medicine (VIFM).
As a consultative service, the patient is discharged back to the referring clinician once the genetic component has been resolved, or an appropriate management plan is drawn up for the referral of at-risk individuals.
Data are maintained in a customised relational database that links individuals from the same family. In recognition of the complexity of genetics and current knowledge limitations in cases where a genetic contribution is suspected but unconfirmed, a plan for periodic file review is prepared, with an electronic reminder system associated with the database. Further, when the clinical implications of a detected genetic variation are unclear, the results are added to the database for future review and reclassification should their interpretation change.
This article presents details of our clinic’s processes and patient population as we move from disease-specific gene testing to next generation sequencing (NGS).
Clinic structure
Our clinic employs a standard operating procedure (Box 1). We have formalised the process of patient review across the departments of genetics and cardiology in our hospital. As many patients travel long distances to visit us, we attempt to provide same-day cardiac testing, before the clinical review. Additional specialised cardiac testing, such as a flecainide test or adrenaline challenge, are provided after the clinical review.
Methods
We extracted data on all patients (n = 1170) who had a first appointment at the CGC between July 2007 and July 2013. This data set included both the proband (the person who triggered the referral to the CGC) and their at-risk relatives. In families where referral followed a death, we describe only the outcomes of the at-risk relatives reviewed.
We extracted the following details for each patient from the CGC database: referral phenotype; number of probands referred (all individuals within this time period were identified as either a proband or an at-risk relative); age at referral; sex and number of at-risk relatives who were screened. Clinical phenotypes are presented in broad diagnostic groups for the purposes of this article.
Approval for the data analysis was provided by the Melbourne Health Human Research Ethics Committee (QA2014096).
Results
Of the individuals seen, 359 (30.7%) were probands, 331 (28.3%) were at-risk family members, and 480 (41.0%) were at-risk family members referred to the CGC following the death of a family member.
Referral diagnosis for individuals seen at the Cardiac Genetics clinic
The distribution of diagnostic categories at referral is presented in Box 2. Inherited cardiac disease in our clinic is categorised into four broad groups: cardiomyopathies, aortopathies, arrhythmias and survivors of cardiac arrest together with families of an SCD individual. In this study:
-
Cardiomyopathy (n = 315) included dilated, hypertrophic, restrictive and arrhythmogenic ventricular cardiomyopathies.
-
Aortopathy (n = 303) included Marfan syndrome, Loeys–Dietz syndrome, familial aortic aneurysm and dissection syndrome, and connective tissue disorders.
-
Arrhythmia disorders (n = 203) included the long-QT, short-QT, Brugada, catecholaminergic polymorphic ventricular tachycardia, mitral valve prolapse, atrial fibrillation, Wolf–Parkinson–White, and sick sinus syndromes.
-
We grouped individuals and families seen after a resuscitated cardiac arrest or SCD (n = 341).
A small number of patients with other diagnoses (n = 8) were seen at the CGC during the study period.
Number of visits
The number of visits per individual during the study period ranged from one to five. Most patients (57.5%) were seen only once. A very small number of individuals (six) were seen on five occasions (Box 3).
Sex
Slightly more women (603) were seen at the CGC than men (565), but the difference was not statistically significant (χ2 test; P = 0.28).
Age
The median age of the study population at the time of patient review was 39 years (range, 13–93 years). Although we operate an adult service, 51 teenagers between 13 and 17 years of age (median age, 16 years) were seen while accompanying family members for a family appointment. Younger teenagers had been offered appointments at a paediatric service, but chose to come to the Royal Melbourne Hospital to avoid being separated from their families. The distribution according to sex and age in each broad diagnostic category is presented in Box 4. There was no statistically significant difference in age between the genders for each diagnostic category (Mann–Whitney) or in the gender spread within each disease category (χ2 test).
Genetic testing
Genetic testing was undertaken in 381 individuals (32.6% of population; median age, 38 years; range, 14–93 years). Of these, five individuals had undergone a total of eight previous genetic tests at another service; the results of these tests were available to us and are included in our summary below. The 788 patients not tested were of similar age (median, 39 years; range, 13–82 years). In 11 individuals (median age, 31 years; range, 15–61 years), a karyotype or microarray test was also ordered, but the results of these tests are not included in this article. Only eight of those who underwent genetic testing were 70 years of age or older (< 0.1% of those tested). The genetic tests undertaken were of two types: mutation detection (in 170 individuals, 44.6%) and predictive genetic tests (in 211 individuals, 55.4%). In total, 47.4% of probands and 26.0% of at-risk family members were offered genetic testing. The apparently low number of at-risk family members offered testing highlights the fact that it has not been the usual practice of the clinic to undertake genetic testing in the absence of a clinical phenotype. For this reason, genetic testing was not undertaken in at-risk relatives when genetic testing of a proband was either inconclusive or uninformative, or when no clinical phenotype had been established for a deceased proband or in an at-risk relative. Of the 211 at-risk family members who underwent genetic testing, this resulted in a new diagnosis for 58 otherwise healthy individuals (27.5% of those tested).
The frequency and type of genetic testing performed in each of the broad diagnostic categories is presented in Box 5. It is notable that our current clinical practice has not expanded to routinely include molecular autopsy in all cases of unexplained death, but is restricted to situations when a diagnosis is suggested by the results of clinical screening in relatives.
The 376 individuals who underwent genetic testing initiated by our service may have had a single gene or a number of genes screened during a single genetic testing episode. In 93.0% of patients, only one genetic testing episode occurred. In 22 individuals, two genetic testing episodes were undertaken; three individuals had three episodes, and one individual had four. A total of 407 genetic testing episodes were undertaken by the clinic during the 6-year period. Pathogenic mutations were detected in 179 individuals (47.6% of those tested), or 15.3% of all patients reviewed by the clinic. These figures underestimate the total number of genetic tests ordered by the clinic, as testing of subsequently deceased probands was not captured in the current data, as previously described.7
Discussion
The CGC aims to confirm or negate a suspected diagnosis of an inherited cardiac condition to allow implementation of a personalised management plan for the affected individuals and their family members. Attending individuals undergo appropriate screening investigations and examination, and genetic testing is offered when indicated, always accompanied by counselling and education. This allows for a targeted risk management strategy or release from screening, as appropriate. For mutation carriers, counselling includes discussion of reproductive options, including both prenatal diagnosis and pre-implantation genetic diagnosis. Clinic staff liaise widely with other specialists, particularly with paediatric cardiologists who provide the clinical care for younger members of identified at-risk families. Translational research is also a focus, and the clinic has been involved with patient groups in education and advocacy, when invited.
The effectiveness of the clinic is facilitated by a formal relationship with the VIFM, whose staff refer at-risk families after potentially genetic cardiac deaths. This includes communication regarding the likely need for genetic testing, which allows the timely storage of biological material.5 To foster robust discussion about the relevance and interpretation of both post mortem findings and family evaluation, the CGC and VIFM meet on a quarterly basis to discuss cases of particular interest or clinical difficulty. This has proved to be critical in allowing detailed discussion of borderline pathological findings where the significance may not be fully appreciated or which could be incorrectly understood to suggest a particular diagnosis.8
The clinical benefit achieved by the simultaneous review of patients by a cardiologist and a clinical geneticist includes the identification of rare diseases and accurate assessment of the utility of genetic testing. This is borne out by the high yield of mutation detection by genetic testing, which highlights the importance of a multidisciplinary clinic and the usefulness of the whole-family approach.9
Published data on genetic testing as part of standard clinical practice of cardiovascular disease in large cohorts is limited, in contrast to genetic testing in a research setting. A Dutch analysis of the results of genetic testing in 6944 individuals identified potential disease-causing mutations in a third of the families seen. The greatest yield was in families with long-QT syndrome (47%) and hypertrophic cardiomyopathy (46%),10 similar to our clinical testing results.
The ultimate intention of the CGC is to prevent adverse cardiac events through early identification and optimal management advice to at-risk individuals. There are currently no long-term data that show improved outcomes are achieved by this approach, and providing these data remains a long-term aspiration of our service. Similarly, although it is anticipated that cost-effectiveness can be achieved, largely by excluding from screening genotype-negative individuals from high-risk families, this remains to be confirmed.
Access to and the application of genetic testing in the CGC has evolved over time. Initially, an iterative approach was adopted, but this was subsequently replaced by small testing panels and, more recently, by a large gene panel (currently including up to 101 known cardiac disease genes). The use of broad panel testing commenced in the clinic during 2013, and the full impact of NGS technologies, in comparison with single mutation detection, has yet to become apparent. With increasing access to large gene panels comes the burden of interpreting multiple genetic abnormalities.11,12 This involves significantly increased time commitment for both the molecular genetics laboratory and for the clinical geneticists and genetic counsellors who inform the patients and their families. These challenges were discussed in a recent review that highlighted the importance of the relationship between the laboratory and clinicians in the delivery of genetic services.13 It is possible that the current targeted panel approach to testing is a transitionary phase before more comprehensive approaches, such as full exome sequencing, are introduced in the clinic. The current methodology has the advantage that, while it generates an increased volume of results that must be managed in the clinic, the risk of completely unanticipated results is minimised.
We anticipate that the use of genetic testing at the CGC will increase in the future, reflecting both its potentially decreasing cost as well as the increased utility of multiple gene testing that is now routine. The currently recommended care model for genetic medicine,9 achieved by a multidisciplinary team working together with the genetics laboratory, provides an effective means for translating advances in genomic medicine into clinical practice.
Box 1 –
Standard operating procedure of the Cardiac Genetics Clinic
|
|
|||||||||||||||
|
Referral |
Referral received |
||||||||||||||
|
|
Discussion with relevant members of the team, as needed |
||||||||||||||
|
Preparation |
Telephone intake appointment by genetic nurse |
||||||||||||||
|
|
Consent(s) gathered for release of information about individuals and family members |
||||||||||||||
|
|
Planning file review (usually by genetic nurse; opportunity to discuss and plan with other staff, if necessary) |
||||||||||||||
|
|
Appointments made for clinic and baseline investigations |
||||||||||||||
|
Clinic appointment day |
In absence of previous screening and wherever possible: same-day resting 12-lead electrocardiogram and/or echocardiogram |
||||||||||||||
|
|
Pre-clinic meeting of whole team (allocation of patients, multidisciplinary decisions about investigation, management planning and genetic testing) |
||||||||||||||
|
|
Consultation with cardiologist/electrophysiologist and clinical geneticist and/or genetic counsellor |
||||||||||||||
|
|
Post-clinic review of plan; data entry by genetic nurse |
||||||||||||||
|
After the appointment |
Follow-up correspondence with referring doctors |
||||||||||||||
|
|
Follow-up correspondence with patient and family |
||||||||||||||
|
|
Coordination of additional special investigations and collation of results |
||||||||||||||
|
|
Follow-up as required (return to clinic if genetic testing ordered; review of individual in cases of clinical uncertainty; discharge back to referring doctor, or assist with referral to appropriate service; discharge from need for care) |
||||||||||||||
|
|
Database notation if future file review is planned, including responsible person and interval |
||||||||||||||
|
|
|||||||||||||||
Box 2 –
Reason for referral of the 1170 individuals seen at the Cardiac Genetics Clinic over a 6-year period

Box 4 –
Number of individuals in each diagnostic category, according to sex and age at time of first clinical review
|
Diagnosis |
Women |
Men |
|||||||||||||
|
Number |
Median age, years (range) |
Number |
Median age, years (range) |
||||||||||||
|
|
|||||||||||||||
|
Cardiomyopathy |
165 |
40 (14–93) |
150 |
40 (14–76) |
|||||||||||
|
Aortopathy |
143 |
38 (14–73) |
160 |
35 (15–71) |
|||||||||||
|
Arrhythmia disorders |
105 |
40 (15–72) |
98 |
38 (14–85) |
|||||||||||
|
SCD or resuscitated cardiac arrest |
188 |
40 (13–82) |
153 |
36 (14–82) |
|||||||||||
|
Other |
3 |
44 (41–56) |
5 |
40 (21–56) |
|||||||||||
|
|
|||||||||||||||
|
SCD = sudden cardiac death. |
|||||||||||||||
Box 5 –
The number and percentage of individuals in each family diagnosis category who underwent genetic testing (mutation detection or predictive testing)
|
|
Cardiomyopathy |
Aortopathy |
Arrhythmia disorders |
SCD or resuscitated cardiac death |
|||||||||||
|
|
|||||||||||||||
|
Total number of patients |
315 |
303 |
203 |
341 |
|||||||||||
|
Genetic tests (% of diagnostic category) |
154 (48.9%) |
101 (33.3%)* |
97 (47.8%)† |
29 (8.5%) |
|||||||||||
|
Median age of tested patients, years (range) |
40 (15–93) |
30 (14–67) |
42 (18–85) |
40 (18–62) |
|||||||||||
|
Mutation detection tests (% of all tests) |
51 (33%) |
65 (64.4%) |
38 (39%) |
16 (55%) |
|||||||||||
|
Number of positive results (% of tests) |
32 (63%) |
33 (51%) |
20 (53%) |
3 (19%) |
|||||||||||
|
Predictive tests (% of all tests) |
103 (66.9%) |
36 (35.6%) |
59 (61%) |
13 (45%) |
|||||||||||
|
Number of positive results (% of tests) |
48 (46.6%) |
10 (28%) |
27 (46%) |
6 (45%) |
|||||||||||
|
Overall positive results (% of all tests) |
80 (51.9%) |
43 (42.6%) |
47 (48%) |
9 (31%) |
|||||||||||
|
|
|||||||||||||||
|
SCD = sudden cardiac death. *Four patients had been tested before their appointment with the Cardiac Genetics Clinic. †One patient had been tested before their appointment with the Cardiac Genetics Clinic. No genetic testing was undertaken in the eight patients with diagnoses outside the four broad categories. |
|||||||||||||||
How changes to the Medicare Benefits Schedule could improve the practice of cardiology and save taxpayer money
The Australian Medicare system is a government-funded fee-for-service system that is highly regarded by the general public. A major advantage of the system is that low-income non-insured patients have ready access to approved ambulatory medical services at little or no cost to them, with public inhospital care provided at no charge. However, a disadvantage is the potential for over servicing. This may occur when new technology or new knowledge lessens or eliminates the indications for a test, without such a development being reflected by a change in the criteria for the particular Medicare Benefits Schedule (MBS) item number. In these circumstances, a medical practitioner may disregard advances in the medical evidence base and continue to practice in the same way, particularly if it is financially advantageous to do so. The examples we discuss in this article reflect this phenomenon. Computed tomography coronary angiography (CTCA), a new, safer and much less expensive technology, should replace invasive coronary angiography (ICA) for the diagnosis of coronary artery disease (CAD), but based on Medicare item reports for 2010–2014,1 this is happening only slowly. Measurement of the fractional flow reserve (FFR) clearly improves the practice of percutaneous coronary intervention (PCI) and saves both money and lives; however, the uptake in Australia has been slow.1 A nuclear stress test has a high radiation burden and is 3.4 times more expensive than a stress echocardiogram,2 yet under the current MBS system it can be ordered by any medical practitioner who may or may not be aware of the cost or the radiation risk.
Invasive coronary angiography
ICA is an expensive procedure ($5187–$6289 per procedure; Appendix 1), with substantial cost to the taxpayer (Box 1). It carries a small risk of serious complications and a radiation burden (5–7 mSv).3 It is a guideline-recommended investigation for patients presenting with troponin-positive acute coronary syndrome.4 In these circumstances, ICA and PCI, if necessary, should be performed by an interventional cardiologist at the same sitting.
ICA is also indicated in symptomatic patients with known stable CAD or with a high probability of CAD who have evidence of myocardial ischaemia of sufficient severity to justify revascularisation with PCI or coronary artery bypass grafting.5 In these circumstances, initial ICA is often performed by a non-interventional cardiologist, and a second ICA and a PCI, if indicated, is then carried out by an interventional cardiologist. This practice is inefficient; the patient and the Medicare system will be billed for two ICAs and a PCI, whereas if the initial ICA had been performed by an interventional cardiologist, only one ICA (and one PCI) would have been charged. Further, in most cases, the decision of a non-interventional cardiologist to refer a patient for PCI after the baseline ICA will be made on visual (anatomical) assessment of the coronary lesion(s), whereas it should be guided by both anatomical and functional assessment.6 The diagnostic accuracy of ICA based on diameter stenosis alone to predict functionally significant coronary artery stenosis (ie, lesions causing ischaemia) is poor.7,8 In the FAME (Fractional flow reserve versus Angiography for Multivessel Evaluation) study, 35% and 80% of coronary lesions seen on ICA with diameter stenosis between 50%–70% and 70%–90%, respectively, were functionally significant by FFR measurement.8 The implication of these findings is that if a patient with stable CAD undergoes ICA for the purpose of assessing suitability for revascularisation, the operator should be capable of performing FFR measurement. As FFR measurement involves instrumentation of the coronary artery with a pressure wire, interventional training is required for its safe performance. This lends further support that ICA is best performed by an interventional cardiologist.
ICA is no longer an appropriate test for the diagnosis of CAD, because it is associated with a low rate of obstructive CAD warranting intervention, even when preceded by an abnormal stress test result.9 It accurately examines the lumen of the coronary artery but does not detect non-obstructive atherosclerotic lesions in the coronary wall that could be a nidus for future coronary events.10 That is, a “normal” ICA finding does not always exclude coronary atherosclerosis.
We suggest that the item numbers for ICA should only be payable if the procedure is performed by an accredited interventional cardiologist in a hospital with accredited PCI facilities.
In cardiology, there is already a precedent for a procedural item number to be available only to an accredited cardiologist. For example, the item number for extraction of a permanent pacemaker lead is only available to cardiologists accredited for that procedure on the advice of the Cardiac Society for Australia and New Zealand. To our knowledge, all public and private hospitals performing PCI have an accreditation process to allow cardiologists to carry out the procedure in their hospital. For new applications, accreditation approval in these hospitals requires evidence that the candidate has undergone specialised training in interventional cardiology and is regarded as competent by his or her supervisors. We suggest that all interventional cardiologists currently accredited to perform PCI be allowed to charge the item numbers for ICA, and that new applications for accreditation be vetted by the Cardiac Society for Australia and New Zealand.
Operator compliance with the indications for ICA could be monitored by examination of an individual cardiologist’s Medicare statistics or, alternatively, by a national cardiac procedures registry. For example, if ICA was only being performed in the setting of troponin-positive acute coronary syndrome or for patients with known CAD and objective evidence of ischaemia not sufficiently controlled with medical therapy, one would expect most patients to require a revascularisation procedure such as PCI or coronary artery bypass grafting. On this basis, the ratio of ICA to revascularisation should be at least less than 2.0 : 1 and preferably in the order of 1.5 : 1. If an individual cardiologist’s statistics fall well outside this range (eg, greater than 2 SDs from that of his or her peers), that cardiologist could be asked to justify the discrepancy.
Computed tomography coronary angiography
Compared with ICA, CTCA is a safer, less invasive and less expensive (the cost to the taxpayer is $622 per angiogram) outpatient investigation and carries a lower radiation burden. The costs of equipping and running a CTCA service in terms of equipment and personnel are far less than those for a cardiac catheterisation laboratory. In regional hospitals without cardiac catheterisation and PCI facilities, the presence and appropriate use of CTCA would allow many patients to be treated locally without the need for transfer to larger centres.
CTCA should be considered as a logical first-line investigation in patients with suspected CAD.11–13 There are three possible outcomes to a CTCA investigation. First, the angiogram may show completely normal results. In such a patient, the likelihood of a coronary event occurring within the next 5 years is extremely low.14,15 Second, the angiogram may show non-obstructive coronary atherosclerosis. In this instance, the patient’s symptoms are unlikely to be caused by myocardial ischaemia. Nevertheless, such patients are at increased risk of future cardiovascular events and require lifestyle advice and possibly anti-atherosclerotic therapy.14,16,17 Third, the angiogram may show obstructive intramural coronary atherosclerotic lesions (or non-evaluable segments as a result of heavy calcifications). Symptomatic patients with these lesions require lifelong anti-atherosclerotic therapy and may benefit from a stress test to determine the presence of ischaemia. CTCA alone is of little or no diagnostic value in patients with pre-existing CAD, because with current technology, routine CTCA is not capable of reliably detecting ischaemia.18
We suggest that the item number for CTCA be payable only if performed in patients without known CAD. For patients whose initial CTCA results are normal, a second CTCA investigation should only be rebatable if it is performed at least 5 years after the first. The imposition of these restrictions would undoubtedly reduce over servicing and help stem the dramatic rise in the use of CTCA.
Stress testing
Stress testing (electrocardiogram based, echocardiogram based or nuclear based) is the non-invasive test of choice for detection of myocardial ischaemia but is a less suitable test for the diagnosis of CAD.19 A standard electrocardiogram stress test is less accurate than either a nuclear stress test or a stress echocardiogram to determine the site and extent of ischaemia. A stress echocardiogram and a nuclear stress test have similar sensitivity for detecting ischaemia but the former has a higher specificity.20 Stress echocardiography is not associated with any radiation exposure but may be technically difficult in patients with unfavourable body habitus. On the other hand, a stress nuclear test is 3.4 times more expensive ($756 v $222) and carries an average radiation burden of 9–11 mSv.3 For these reasons, we suggest that the item number for a nuclear stress test be payable only if ordered by a physician and only if a stress echocardiogram is considered unsuitable for technical reasons.
Percutaneous coronary intervention
ICA with a view to PCI at the culprit lesion, if technically suitable, is a guideline recommendation for patients with acute coronary syndrome.4 In stable CAD, the benefit from PCI with optimal medical therapy is less certain compared with medical therapy alone.21 Furthermore, stenting of non-ischaemic coronary lesions leads to higher rates of mortality and myocardial infarction.22 A coronary lesion can be assumed to be causing ischaemia only if there is > 90% stenosis in a major coronary artery or if it is a single lesion in a coronary vessel supplying an area of myocardium identified as ischaemic on stress testing. All other coronary lesions should not be stented in stable CAD unless the FFR is less than 0.8. Use of FFR in this manner has been shown to reduce stent insertions, improve outcomes and lower health costs.23,24 According to Medicare item reports for 2013–2014,1 only 16% of cases of PCI were associated with FFR. The implication of this finding is that, in Australia, many patients must be undergoing PCI procedures that are potentially detrimental to their health.
We suggest separate MBS item numbers for PCI for troponin-positive acute coronary syndrome and for PCI for stable CAD, thus allowing easier evaluation of the Medicare statistics of an individual practitioner. The item number for PCI for stable CAD should only be payable if one of three conditions is satisfied: (i) a stenosis >90% in a coronary vessel >2 mm in diameter; (ii) a single lesion in a vessel supplying an area of myocardium identified as ischaemic on stress testing; or (iii) a coronary lesion associated with an FFR less than 0.8.
Overall savings resulting from our proposed changes
The overall savings resulting from these changes are summarised in Box 2. Medicare statistics along with data from the Australian Commission on Safety and Quality in Health Care25 were used to calculate the ratio of ICA to revascularisation and the cost to the taxpayer of unnecessary ICA (defined as in excess of a ratio of 1.5 : 1; Appendix 2). Applying this ratio to the four patient groups discussed, taxpayers could have saved $233.5 million and private health insurance companies $139.8 million in 2013–2014.
If our suggested changes to PCI were to occur, the annual savings to the Australian health budget would be in the order of $4 million.24 Changes for CTCA would be cost neutral in the short term but would save costs in the long term (Appendix 2).
In 2013–2014, 77 564 nuclear stress tests were charged to Medicare (cost per test, $756). It is likely that at least 75% of these patients could have had a less expensive stress echocardiogram (cost per test, $222) as an alternative. Doing so would have saved over $30 million of the Medicare budget.
We believe that these relatively simple changes to the MBS would improve the practice of cardiology (Box 3) and result in substantial savings to the health budget (Box 2). Undoubtedly, some cardiologists will consider the suggested changes to be an unwelcome interference with their practice. The counter argument is that as funders of Medicare, the government has a right and a duty to spend public money prudently.
In 2013–2014, the ratio of ICA to revascularisation was substantially higher in the private compared with the public system (3.1 v 2.3; Appendix 2). The likely explanation relates to the effect of fee-for-service on the provision of ICA.
A potential disadvantage of performing PCI at the same sitting as the initial ICA is that the patient will be denied the opportunity for surgical consultation. However, in light of recent evidence indicating the clear superiority of coronary artery bypass grafting over PCI for patients with complex multivessel disease or with diabetes with multivessel disease,26,27 we believe the need for multidisciplinary discussion to determine the best revascularisation option will be infrequent. We consider our recommended ratio of ICA to revascularisation of 1.5 : 1 or less to be sufficiently elastic to accommodate this possibility without compromising patient care.
In summary, we believe these relatively simple changes to the MBS would result in improved evidence-based cardiology practice and substantial savings to the health budget in an ever-increasingly constrained fiscal climate.
1
Cost to the taxpayer of unnecessary invasive coronary angiography (ICA), 2013–2014
|
|
ICA to revascularisation ratio |
No. of unnecessary cases |
Cost per unnecessary case |
Cost per year |
|||||||||||
|
|
|||||||||||||||
|
Public inpatient |
2.3 : 1 |
23 060 |
$5773 |
$133.13m |
|||||||||||
|
Private in public |
2.7 : 1 |
2986 |
$4964 |
$14.82m |
|||||||||||
|
Private inpatient |
3.1 : 1 |
38 259 |
$2199 |
$84.13m |
|||||||||||
|
Non-insured outpatient |
2.4 : 1 |
1871 |
$759 |
$1.42m |
|||||||||||
|
Total cost |
|
|
|
$233.5m |
|||||||||||
|
|
|||||||||||||||
2
How much money could be saved?
|
Measure |
Potential annual savings |
||||||||||||||
|
|
|||||||||||||||
|
Reducing invasive coronary angiography to revascularisation ratio to 1.5 : 1 |
$233.5m |
||||||||||||||
|
Limitations to computed tomography coronary angiography |
Cost neutral |
||||||||||||||
|
Reducing nuclear stress tests |
$30.1m |
||||||||||||||
|
More use of fractional flow reserve |
$4.0m |
||||||||||||||
|
Total |
$267.6m |
||||||||||||||
|
|
|||||||||||||||
3
How our proposed changes to the Medicare Benefits Schedule could improve cardiology practice
- More judicious use of invasive coronary angiography = less complications, less radiation exposure, less waste of catheter laboratory resources.
- More judicious use of computed tomography coronary angiography = earlier diagnosis of coronary artery disease, better prognostic assessment, lifestyle modifications and medical therapy where appropriate.
- More judicious use of nuclear stress test = less radiation burden.
- Greater use of fractional flow reserve-guided percutaneous coronary intervention = less inappropriate percutaneous coronary intervention and less myocardial infarction and death.
Transplantation of the heart after circulatory death of the donor: time for a change in law?
Australia has an increasing shortfall in transplantable hearts. Over the past decade, the number of all donors per million population increased from 10.0 in 20051 to 16.1 in 2014.2 However, the number of heart donations per million population over the same period has declined slightly from 3.8 to 3.4, with an annual average of 3.3.3 Procurement of organs has always been conducted according to the dead donor rule — that is, after death of the donor — but this practice is being challenged.
The law defines death in all Australian jurisdictions (eg, in s 41 of the Human Tissue Act 1982 [Vic]) as either “irreversible cessation of all functions of the brain” (brain death) or as “irreversible cessation of circulation of blood in the body” (circulatory death), but it does not define irreversible or how to determine irreversibility (Box). Exceptionally, circulatory death is not defined in Western Australian legislation.
Although the procurement of organs such as livers, kidneys and lungs is permitted after either brain death or circulatory death according to Acts in all jurisdictions, the procurement of hearts has traditionally only been from brain dead donors with functioning hearts. The definition and diagnosis of brain death is not without controversy4,5 and may explain in part why more reliance is being placed on circulatory death, which reduces availability of hearts.6,7 Alternatively, organ procurement from patients after circulatory death may be perceived as more realisable than after brain death. Indeed, circulatory death as the source of solid organs has increased from 10% of 204 donors in 2005 to 28% of 378 donors in 2014.1,2 More total organs have been procured (from 726 to 1193) but the number of hearts has increased only slightly from 72 of 204 donors (35%) to 79 of 378 donors (21%) over the same period.1,2 Only 39 hearts were procured from 189 donors (21%) during the first 6 months of 2015.8
Organ donation and procurement after circulatory death
In the practice of organ donation after circulatory death (DCD), life-sustaining treatment such as mechanical ventilation is commonly withdrawn because of a devastating neurological injury that has not progressed to brain death. The withdrawal of treatment from the donor is staged to facilitate organ transplantation to recipients. The procurement of organs is specified in the Australian national DCD protocol published by the Organ and Tissue Authority9 and enacted through the DonateLife network. It is not declared why the heart is not included in the protocol’s list of organs that may be procured.
Expeditious organ procurement may be commenced after death has occurred — defined in Australia as 2–5 minutes after cessation of the donor’s circulation,9 and after 2 minutes in United States.10 The donor’s arrested heart has not usually been procured. However, to increase the availability of hearts, routine procurement after circulatory death is proposed but not yet sanctioned in the current national DCD protocol.9 It is probable that the protocol is under review given that the Organ and Tissue Authority lauded St Vincent’s Hospital in Sydney, where two adults were transplanted with hearts procured after circulatory death in 2014.11 In those cases, the donor hearts were reanimated and kept beating and warm inside a container (ex-vivo Organ Care System, TransMedics) pending transplantation.12
This practice poses ethical,13 legal and medical problems. Foremost of the medical difficulties is the poor condition of the procured heart after it has ceased to circulate blood in the donor. However, the heart can be resuscitated with the aid of extracorporeal techniques, as was performed for the two adult recipients at St Vincent’s Hospital.11,12
Heart transplantations after cardiac death have been performed in three infants in North America14 but were followed by consternation and a medical call for a moratorium on all organ procurement after cardiac death and the accusation that doctors and hospitals were biased towards organ procurement.15 The basis for the opposition was that the infants may not have been dead and possibly were conscious at the time of organ procurement. Of the three infants, one was declared dead 3 minutes after cessation of cardiocirculatory function before the process of organ procurement was initiated, and two were declared dead after 75 seconds.14
Medical interpretation of the legal definition of death
The source of the problem of heart transplantation after circulatory death is the medical interpretation of the legal definition of circulatory death. From a medical point of view, in other contexts, death is not necessarily defined by cessation of the circulation, unless it is of sufficient duration to result in brain death. It is not rare, for example, to be able to resuscitate a victim from a short duration cardiac arrest with complete neurological recovery or to sustain the circulation of a patient for lengthy periods by an extracorporeal circulation with subsequent intrinsic cardiac recovery.
The fact that a transplanted heart can function and sustain life in a recipient must mean that the circulation of the donor is never ceased irreversibly and therefore that the donor of the heart is never dead until his or her heart is removed.
The question is thus posed — how is it possible to procure the heart of a donor under the premise of circulatory death and yet expect it to sustain life in a recipient? Put differently, should the procurement of the heart be a criminal offence in such cases, because its procurement is the cause of death of the donor?
A possible argument to justify heart procurement for transplantation after circulatory death is that legal irreversible cessation of the circulation may be interpreted medically as “will not be resuscitated” rather than “cannot be resuscitated”. However, that interpretation does not appear to be open to the medical profession. From a legal point of view, the meaning of legislation is governed by statute law. In the jurisdiction considered here, it is the Interpretation of Legislation Act 1984 (Vic) and Acts Interpretation Act 1901 (Cwlth) that apply, but these are not helpful in defining “irreversible”.
If a word or phrase is not defined in an Act, resort is made to common law interpretations, which may be described as literal or purposive. The literal interpretation of legislation was defined by Justice Higgins in the High Court of Australia as:
The fundamental rule of interpretation, to which all others are subordinate, is that a statute is to be expounded according to the intent of the Parliament that made it; and that intention has to be found by an examination of the language used in the statute as a whole. The question is, what does the language mean; and when we find what the language means, in its ordinary and natural sense, it is our duty to obey that meaning, even if we think the result would be inconvenient or impolitic or improbable.16
The literal approach may be too restrictive if a word or phrase has more than one meaning. In such instances, legal resort is made to a reputable dictionary — in Australia, usually the Macquarie Dictionary. Since that dictionary defines “irreversible” as “not reversible; that cannot be reversed”, a legal and hence medical interpretation of irreversible cannot logically be “will not be resuscitated”. Doctors cannot simply redefine the meaning of words in legislation to suit their practice.
Notwithstanding that the natural and ordinary meanings of words are the starting points in interpreting Acts,17 a purposive interpretation may be considered when a literal approach produces ambiguity or inconsistency (Acts Interpretation Act, s 15AA). Such an interpretation would be one that best achieves the purpose or object of the Act, whether expressly stated or derived from the content of the Act.
In Victoria, the stated aim of the Human Tissue Act is to make provision for removal of human tissue for transplantation and, among other aims, to provide a definition of death. The purpose of defining death is not declared but s 26 of the Act allows a designated officer and medical officers to remove tissue for transplantation only when the proposed organ donor has fulfilled the definition of death under s 41. In other words, a purpose of the Act is to prevent tissue and organ procurement from a donor who is not dead. Thus, heart procurement for transplantation under the practice of DCD is not possible under either a literal or purposive interpretation of the Act.
A similar problem with the interpretation of legislation has occurred in the United States, where death is defined in the Uniform Determination of Death Act 1982, on which Australian legislation has been modelled. The US Act also defines circulatory death as “irreversible cessation of circulatory and respiratory functions”. In a purposive approach, Bernat18 has proposed that as doctors diagnose death by permanent cessation of circulatory and respiratory functions, this satisfies the requirements of death statutes and does not violate the dead donor rule. Bernat also proposes that “permanence is a perfect surrogate indicator for irreversibility” and thus permits heart donation after DCD. This argument is similarly not sustainable. The Macquarie Dictionary defines “permanent” as “lasting or intended to last indefinitely; remaining unchanged; not temporary; enduring; abiding”. Clearly, a heart that has ceased functioning in a donor and functions later in a recipient has not ceased functioning irreversibly or permanently. Moreover, permanent cessation of the circulation is not the legal definition of death, and the concept, wrong that it is, is similarly not available to doctors to justify heart transplantation after circulatory death.
Another putative justification for heart procurement after DCD is that, whereas the heart may have stopped irreversibly in the donor’s body, it is able to function in that of the recipient. This is also a spurious argument, because the only reason that the heart stops in the donor is the elective, and hence reversible, withdrawal of life-sustaining treatment such as mechanical ventilation. The heart had obviously been functioning well in the donor’s body before its procurement.
Possible solution
This potential problem of heart procurement being the cause of the donor’s death arises because death has been mistakenly defined in the legal sense as cessation of the circulation, without any reference to brain function. A possible alternative would be to retain the present definition of brain death as irreversible cessation of all function of the brain, but to omit the requirement for irreversibility in the definition of circulatory death and to redefine it as cessation of circulatory function with cessation of higher brain function. Under this proposition for the redefinition of circulatory death for the purpose of transplantation, procurement of a heart for the purpose of its transplantation could proceed without legal risk and without risk of retained consciousness of the donor.
Conclusion
Organ transplantation is ethical whether after brain death or circulatory death, and it is proper to maximise organ procurement, but only as permitted by law. We have shown that heart transplantation after DCD does not conform to present statute law. When the way forward is not clear in a medicolegal conundrum such as this one, legislation needs to be refined. Otherwise, as some legal academics have argued,19 procurement of a heart after cardiac death for transplantation under present legislation does not conform to the dead donor rule. This may be a potential criminal offence, an accusation that may need to be made in order to encourage law reform. Alternatively, the dead donor rule, which would arguably be violated in heart transplantation after circulatory death, needs societal, legal and medical debate followed by revision or abandonment.13 Otherwise, Australia’s improving organ donor program is at risk of adverse publicity and damage if doctors, hospitals and our organ procurement agencies are perceived as procuring organs from patients not legally dead.
Australian statute law governing procurement of organs for transplantation
|
Jurisdiction |
Act |
Provision |
|||||||||||||
|
|
|||||||||||||||
|
NSW |
Human Tissue Act 1983 |
s 33 |
|||||||||||||
|
Qld |
Transplantation and Anatomy Act 1979 |
s 45(1) |
|||||||||||||
|
SA |
Transplantation and Anatomy Act 1983 |
s 24(2) |
|||||||||||||
|
|
Death (Definition) Act 1983 |
s 2 |
|||||||||||||
|
Tas |
Human Tissue Act 1985 |
s 27A |
|||||||||||||
|
Vic |
Human Tissue Act 1982 |
s 41 |
|||||||||||||
|
WA |
Human Tissue and Transplant Act 1982∗ |
s 24(2) |
|||||||||||||
|
ACT |
Transplantation and Anatomy Act 1978 |
s 45 |
|||||||||||||
|
NT |
Transplantation and Anatomy Act 2014 |
s 23 |
|||||||||||||
|
|
|||||||||||||||
|
∗Note: circulatory death is not defined in Western Australian legislation. |
|||||||||||||||

 more_vert
more_vert