Why provide life expectancy information? An integral part of patient-centred cancer care is ensuring that information, communication and education provided to patients meets their needs, preferences and values.1 Between 50% and 70% of patients with cancer want numerical estimates of their life expectancy.2–6 Assessing and responding to patient preferences about life expectancy information is therefore a necessary component of patient-centred care, and can assist patients in making informed and effective decisions about their care. Misperceptions of life expectancy by patients, however, can also influence aspects of care, such as decisions about continuing life-prolonging treatments that may diminish their quality of life.7,8
Discussing life expectancy is a complex task. Clinicians must 1) establish how much, and in what detail patients want to know; 2) offer timely information that facilitates decision making about treatment and informed consent; 3) provide information consistent with patient preferences; 4) communicate the limitations inherent to prognoses; 5) present information in formats that aid understanding; and 6) ensure that information is communicated sensitively.6,9 Self-reports by patients about their awareness and understanding of what they have been told about life expectancy arguably comprise the gold standard measure of quality in this area.10,11
Is there concordance between patient preferences and experiences of discussions of life expectancy? The proportion of patients with cancer who reportedly discuss life expectancy is variable. Using direct observation, one study found that 58% of incurable oncology patients were told about life expectancy.12 Lower rates of disclosure (27%–53%) have been reported in studies based on patient self-reports.6,13 While discrepancies between preferences and experiences regarding prognosis information have been reported,6,14 few studies have focused specifically on life expectancy information. One investigation found that 47% of patients who wanted life expectancy information did not receive it, and 4% had received information they did not want.5 The preferences of patients receiving radiation therapy for cancer in Australia regarding who should initiate life expectancy discussions (the patient or their doctor) and their actual experiences were not aligned in 40% of instances.13
Previous studies of this question have been limited by convenience samples or low response rates (24% in one study5), or by including only participants with a single cancer type from a single treatment centre.4 The degree to which these data can be generalised to all cancer patients is therefore questionable. The literature indicates that patients’ preferences and experiences of prognosis discussions may vary according to their age, sex, marital status, ethnic background, education, disease status, time since diagnosis, cancer type and psychological wellbeing.4–6,15,16 Given the emphasis on reducing disparities in health care,1 it is important to explore factors associated with misalignment of patient preferences and experiences, and to identify subgroups who are less likely to receive the desired information.
Our multisite study aimed to identify:
-
the proportion of patients who received their preferred level of life expectancy information; and
-
the sociodemographic, clinical and psychological factors associated with patients’ perceptions of receiving too little, too much, or the desired amount of life expectancy information.
Methods
Patient sample
Eligible medical oncology treatment centres were those providing care for at least 400 new cancer patients each year, and were nominated by state-based research representatives to reflect the relative distribution of public, private, metropolitan and regional hospitals across each of the six Australian states. Invitations were sent to the 51 nominated centres by representatives on behalf of the research team.
Eligible patients had a confirmed cancer diagnosis, were attending the clinic for their second or subsequent appointment (to ensure that patients had experienced cancer care at the centre before answering questions about this treatment), were at least 18 years old, and were able to read and understand English. They were judged by clinical staff to be physically and mentally able to give informed consent and to complete the survey.
Informed consent was obtained by a researcher or clinic staff member by consecutively approaching eligible patients while they waited for their outpatient appointments. To assess consent bias, those who withheld consent were asked to provide their age and sex (this was not done for consenters). Consenting patients were asked to complete a pen-and-paper survey, either in the clinic or at home. Non-responders were sent reminder letters 2–3 weeks and 5–6 weeks after recruitment.
The survey instrument
Development of the measure
Survey items (outcome and associate items) were distributed to a sample of consumer advocates for qualitative feedback on item comprehensibility and relevance. Items were then piloted with 324 patients, and then revised to improve their quality and acceptability. The revised survey (detailed below) was completed by the participants in our study.
Life expectancy item
Participants were asked “Which of the following best describes your experience of discussions with your cancer doctor(s) about how cancer may affect the length of your life (your life expectancy)?” A question stem and five response options were provided: “My cancer doctor(s) at this hospital has discussed or given me …”:
-
More information than I wanted about my life expectancy;
-
All the information that I wanted about my life expectancy;
-
Some of the information that I wanted about my life expectancy;
-
None of the information that I wanted about my life expectancy; or
-
No information about life expectancy, but I haven’t wanted information.
Associate variables
All associate variables were obtained from patient self-reports. Sociodemographic items included sex, age, marital status, education (a proxy for socioeconomic status17), and ethnic background (for participants born outside Australia or who identified as being Aboriginal and/or Torres Strait Islander).
Clinical items included the patient’s most recent cancer type, stage of cancer at diagnosis, current remission status, time since diagnosis, and main reason for visit to the clinic on the day of recruitment.
Psychological wellbeing was measured with the Hospital Anxiety and Depression Scale (HADS), a 14-item survey with two subscales, anxiety and depression. Each item is rated on a four-point scale; scores range from 0 to 21 for each subscale. A mean cut-off score of 8 on each subscale optimises its sensitivity and specificity as a screening instrument.18
Statistical analysis
Stata/IC 11.1 (StataCorp) was used for all analyses. Consent bias (age, sex) was assessed with χ2 analyses. Frequency tables were calculated for each of the five life expectancy response options. A multinomial logistic regression, adjusted for clustering of results within centres by jacknife estimation, examined factors associated with the alignment of patient preference and experience. Key variables were selected a priori. Four preference–experience outcome categories were generated:
-
too much information (“More information than I wanted”);
-
too little information (“Some of the information I wanted” or “None of the information I wanted”);
-
no information wanted or received (“No information about life expectancy, but I haven’t wanted information”); and
-
received all the information wanted (“All the information that I wanted”).
The fourth category was the reference category. While the third and fourth categories both included patients whose preferences and experiences were aligned, the large sample size offered an opportunity to explore these groups separately. Odds ratios (ORs) with 95% confidence intervals and the results of adjusted Wald tests are reported.
Ethics approval
The University of Newcastle Human Research Ethics Committee and the ethics committees of the participating health services approved the study (ref. H-2010-1324).
Results
Of 51 medical oncology treatment centres that were approached, 14 consented to participate in the study (27% consent rate). Two consenting centres did not participate: one had an ethics process that was too expensive, and the other chose not to participate after consenting. Eleven of the participating treatment centres were public medical centres; nine were located in metropolitan and three in regional areas. At least one centre from each Australian state participated. Patients from 11 centres received the survey items on life expectancy during the recruitment period.
Patient sample
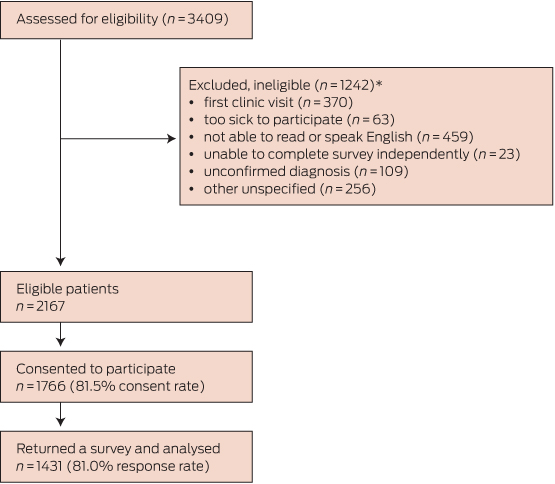
Of the 2167 eligible patients, 1431 returned a survey (Box 1). There were significant sex (χ2[1] = 12.3, P < 0.001; more women) and age differences (χ2[5] = 13.3, P = 0.02; more older patients) compared with those who withheld consent to participate. The age of those who initially consented but later did not respond could not be determined. Box 2 summarises the characteristics of the 1431 participants and the non-consenters.
Do patients receive their preferred level of information about life expectancy?
Of the 1431 responders, 1361 (95.1%) completed the life expectancy item of the survey. Responders did not differ from non-responders with respect to age (χ2[5] = 7.1, P = 0.21), sex (χ2[1] = 2.2, P = 0.14), treatment centre attended (χ2[10] = 13.8, P = 0.18), cancer type (χ2[4] = 4.4, P = 0.36) or remission status (χ2[2] = 0.2, P = 0.91), but participants who did not complete the item were less likely to have had advanced cancer at diagnosis (χ2[2] = 10.7, P = 0.005).
As summarised in Box 3, 72% of patients received the information that they desired; that is, 50% received all the information that they wanted, and 22% neither wanted nor received any information. A mismatch between preferences and experiences was reported by 388 patients (28%), of whom 24% reported not receiving enough information and 4% reported receiving too much.
After adjusting for clustering within treatment centres, no variation between institutions in the perception of life expectancy discussions was identified (post hoc χ2[10] = 35.9, P = 0.21).
What factors are associated with the alignment of preferences and experiences?
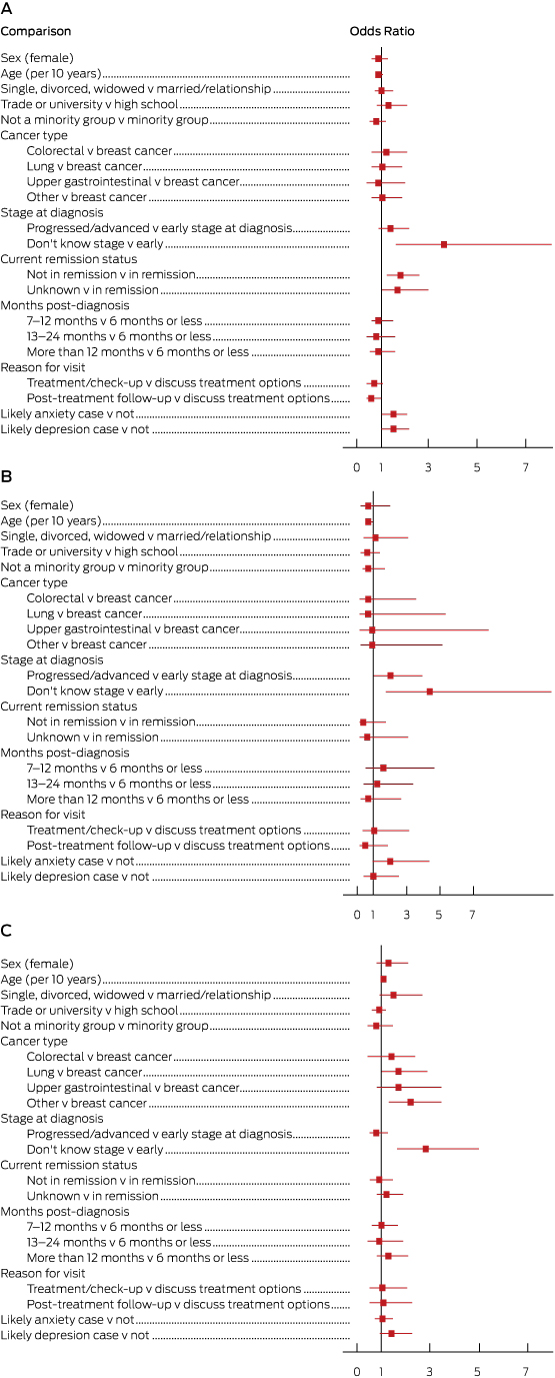
Box 4 and the Appendix present the results of our multinomial logistic regression. The reference group included those who received the desired amount of information about life expectancy. The odds of receiving too little information were greater for patients not in remission (OR, 1.8; 95% CI, 1.2–2.6), who did not know their cancer stage at diagnosis (OR, 3.6; 95% CI, 1.6–8.1), or who were likely to have anxiety (OR, 1.5; 95% CI, 1.0–2.1) or depression (OR, 1.5; 95% CI, 1.0–2.2). Younger patients (OR, 1.4; 95% CI, 1.0–2.0), those with a more progressed cancer (OR, 2.0; 95% CI, 1.0–4.0) or who did not know their stage at diagnosis (OR, 4.4; 95% CI, 1.7–11.8) were more likely to receive too much information. Older patients (OR, 1.1; 95% CI, 1.0–1.2) and those who did not know their stage at diagnosis (OR, 2.8; 95% CI, 1.6–5.0) were more likely to report that they neither wanted nor received information.
Discussion
Do patients receive their preferred level of information about life expectancy?
Almost three in four patients (72%) reported receiving the desired level of information about life expectancy: half of our sample (50%) reported receiving adequate levels of information, while 22% neither wanted nor received any information. This confirms previous findings that patients have different life expectancy information needs.2–5
The degree to which patients receive life expectancy information that aligns with their preferences has not improved dramatically.5 Discordant preference–experience outcomes most commonly involved patients receiving less information than they desired. Clinicians may avoid discussing prognosis and life expectancy in day-to-day practice because of uncertainty about the accuracy of estimates, a perceived lack of time, or concerns about their ability to deal with their patients’ emotions.6,19 Clinicians may wait for patients to request life expectancy information before providing it, or discuss it in ways that may be difficult for some patients to understand.5,6,19 Given that a minority of patients (4%) received more information about life expectancy than they wanted, and a substantial minority prefer to receive no information (22%), it is not appropriate to provide comprehensive information to all patients. Clinicians should take an individualised approach when providing life expectancy information.
Who gets too little information?
Patients not in remission were more likely to receive less life expectancy information than desired. As communicating a poor prognosis is complex, patients may not always understand such information in a manner that aids their decision making.11 As a consequence, clinicians should ensure they have the necessary skills to sensitively provide such information to patients who wish to be informed.20
Elevated anxiety and depression scores were associated with receiving too little information about life expectancy. Clinicians may withhold information from patients whom they perceive to be anxious or depressed, particularly if the prognosis is poor.10 On the other hand, receiving less information than desired may itself increase anxiety and depression.
Who gets too much information?
Patients who received too much life expectancy information were younger and reported having more advanced cancer at diagnosis. This finding may reflect assumptions by clinicians that younger patients will want life expectancy information. It may be especially important for those with a poor prognosis in order to guide decisions about treatment. Our findings may also indicate that clinicians assume that those with a poor prognosis want to receive this type of information.
Who neither wants nor receives information?
Consistent with the results of previous research, patients who neither wanted nor received life expectancy information were significantly more likely to be older.15,21,22 Older patients frequently report higher levels of satisfaction with cancer care.23 Younger patients are more likely to request and receive information about the prognosis,5,20 possibly reflecting increasing expectations of being involved in treatment decisions, and also the potential value of this information for assisting them regain some degree of control over their life plans.6
Stage of cancer at diagnosis unknown
One of the strongest and most consistent associate predictors was not knowing the stage of cancer at diagnosis (9% of sample). These patients had greater odds of reporting that they had received too little or too much information, or that they neither wanted nor received any information. This may reflect a general dissatisfaction with the providing of information, or that cancer diagnosis staging and prognosis was unknown to their doctors. Alternatively, it could reflect a generalised communication problem or the patient’s difficulty in understanding the clinical aspects of their diagnosis. Particular patient groups may need additional support to ensure their accurate understanding of clinical information.
Strengths and limitations of our study
While women were overrepresented and younger patients underrepresented in our sample, this multisite study is the largest and most representative to examine the research question. We are unable to determine whether cancer type profile and other clinical characteristics of the sample are representative of these parameters for the overall population of patients with cancer. While patient reports are the gold standard for assessing patient-centred care,1 they can be influenced by recall bias. Clinical characteristics were assessed by patient self-report, which may be less accurate than data obtained from medical or registry records. However, the clinical items in the survey were pilot-tested and structured to facilitate understanding by patients (lay terms, the use of examples) and to increase accuracy. The participants’ levels of health literacy, which is correlated with information-seeking behaviours and understanding provided information, were not measured, as the study aimed to assess the experiences and preferences of all patients who attended the treatment centres. The preferences of patients and their experiences may change over time as their circumstances change, so that future research should apply a longitudinal study design.
Clinical implications
To improve care delivery, health care teams should regularly collect patient feedback on the quality of care at both the patients’ and the institutional levels. Clinicians could ask patients whether they have received and understood the information about life expectancy provided to them. Enquiring about their information preferences should occur across several consultations to allow patients to process information and to formulate questions.9 At the institutional level, regular feedback about life expectancy information could be incorporated into patient experience surveys. As this study found, asking patients about this topic is practicable and acceptable, but current patient experience surveys do not routinely explore this aspect of care.
It might be expected that delivering information about life expectancy would be a matter of institutional policy. However, the lack of variation across centres suggests that these discussions are regulated by individual clinicians, rather than by policies or monitoring processes. To improve care for the 28% of patients whose preferences and experiences were not aligned, institution-wide policies and routine feedback should be considered.
Conclusions
Discussing life and death is emotional for patients, their families and their friends. That fact that 28% of cancer patients do not receive the level of information about life expectancy that they desire highlights the difficulties associated with discussing this sensitive topic. While not all patients want to receive detailed information, discordance was more often the result of patients wanting more rather than less information. The first step for clinicians should therefore be to ask whether the individual patient wants to know this information, in what format, and at what level of detail (eg, estimated life expectancy, cancer staging, prospects for cure, aim of cancer treatments). Australian consensus guidelines9 are available to assist clinicians in communicating information about life expectancy, including advice about using generic communication skills (eg, body language and active listening), and about clarifying the questions of patients and caregivers and addressing their information needs in an ongoing conversation over time. While the onus of responsibility remains with the clinician to ensure that life expectancy discussions occur in accordance with patient preferences, question prompt lists have been identified as helpful for enabling patients to obtain the information they desire.24
Box 1 –
Patient recruitment and data collection process
Box 2 –
Sociodemographic and clinical characteristics of the study participants, and age and sex data for non-consenting patients∗
|
|
Study sample
|
Non-consenters
|
|
|
Total number
|
1431
|
401
|
|
Sex
|
|
|
|
Male
|
601 (42%)
|
200 (52%)
|
|
Female
|
828 (58%)
|
184 (48%)
|
|
Missing data
|
2
|
17
|
|
Age at diagnosis, years (mean ± SD)
|
62.5 ± 12.4
|
|
|
18–34 years
|
33 (2%)
|
16 (4%)
|
|
35–44 years
|
88 (6%)
|
17 (4%)
|
|
45–54 years
|
242 (18%)
|
57 (15%)
|
|
55–64 years
|
395 (29%)
|
120 (31%)
|
|
65–74 years
|
413 (30%)
|
100 (26%)
|
|
≥ 75 years
|
206 (15%)
|
76 (20%)
|
|
Missing data
|
54
|
15
|
|
Marital status
|
|
|
|
Married or in a relationship
|
906 (65%)
|
|
|
Single, divorced or widowed
|
489 (35%)
|
|
|
Missing data
|
36
|
|
|
Education
|
|
|
|
Primary school
|
97 (7%)
|
|
|
High school
|
600 (43%)
|
|
|
Trade or university
|
637 (46%)
|
|
|
Other
|
48 (3%)
|
|
|
Missing data
|
49
|
|
|
Minority group
|
|
|
|
Aboriginal and/or Torres Strait Islander
|
19 (1%)
|
|
|
Not born in Australia
|
438 (31%)
|
|
|
Neither
|
935 (67%)
|
|
|
Missing data
|
39
|
|
|
Cancer type
|
|
|
|
Breast
|
454 (33%)
|
|
|
Colorectal
|
236 (17%)
|
|
|
Lung
|
140 (10%)
|
|
|
Upper gastrointestinal
|
130 (9%)
|
|
|
Prostate
|
78 (6%)
|
|
|
Other urogenital
|
75 (5%)
|
|
|
Haematological
|
60 (4%)
|
|
|
Gynaecological
|
49 (4%)
|
|
|
Other
|
154 (11%)
|
|
|
Missing data
|
55
|
|
|
Stage of cancer at diagnosis
|
|
|
|
Early
|
818 (61%)
|
|
|
Advanced
|
408 (30%)
|
|
|
Don’t know
|
117 (9%)
|
|
|
Missing data
|
88
|
|
|
Current remission status
|
|
|
In remission
|
409 (30%)
|
|
Not in remission
|
559 (41%)
|
|
Don’t know
|
411 (30%)
|
|
Missing data
|
52
|
|
Months since cancer diagnosis
|
|
|
Less than 6 months
|
425 (30%)
|
|
6–12 months
|
260 (19%)
|
|
13–24 months
|
244 (17%)
|
|
More than 24 months
|
473 (34%)
|
|
Missing data
|
29
|
|
Reason for clinic visit
|
|
|
To discuss treatment options
|
117 (9%)
|
|
To receive treatment or check-up during treatment
|
801 (61%)
|
|
Post-treatment follow-up
|
405 (31%)
|
|
Missing data
|
108
|
|
Treatment received to date†
|
|
|
Surgery
|
977 (70%)
|
|
Chemotherapy
|
113 (81%)
|
|
Radiotherapy
|
664 (51%)
|
|
Hormonal manipulation
|
312 (24%)
|
|
Biological therapies
|
146 (11%)
|
|
Treatment centre
|
|
|
A 136 (10%) G
|
105 (7%)
|
|
B 111 (8%) H
|
155 (11%)
|
|
C 159 (11%) I
|
163 (11%)
|
|
D 101 (7%) J
|
140 (10%)
|
|
E 86 (6%) K
|
158 (11%)
|
|
F 117 (8%)
|
|
|
|
∗ Percentages in this table exclude missing data. † Patients may have received more than one treatment type.
|
Box 3 –
Concordance between patient preferences and experiences in discussions of life expectancy∗
|
Category
|
Response option
|
Number of patients
|
% (95% CI)
|
|
|
Too much information
|
More information than I wanted about my life expectancy
|
56
|
4% (3%–5%)
|
|
Too little information
|
|
332
|
24% (22%–27%)
|
|
|
Some of the information I wanted about my life expectancy
|
258
|
19%
|
|
|
None of the information I wanted about my life expectancy
|
74
|
5%
|
|
No information wanted nor received
|
No information about life expectancy, but I didn’t want information
|
298
|
22% (20%–24%)
|
|
The right amount of information
|
All the information that I wanted about my life expectancy
|
675
|
50% (47%–52%)
|
|
|
∗Data was missing for 70 patients (4.9%), so that sample size for this table is 1361 patients.
|
Box 4 –
Sociodemographic, clinical and psychological factors associated with level of life expectancy information received. A, Too little information received; B, Too much information received; C, Information neither wanted nor received

 more_vert
more_vert