More Australian men and women die from lung cancer than from any other cancer.1 It is the fourth most common neoplasm in both men and women, and in 2014 more than 11 000 people were diagnosed with lung cancer in Australia.1 The 5-year survival rate is 14%;1 the median survival time for patients with non-small cell lung cancer (NSCLC) is 6.9 months, and for small cell lung cancer (SCLC) it is 7.2 months.2
A recent Victorian study found that only 30% of patients with NSCLC received treatment with curative intent, and only 33% were discussed in a multidisciplinary team meeting.2 While 26% of patients presented with stage III disease, only 8% had invasive staging of the mediastinum, highlighting a potential discordance in staging of the cancer. The study did not assess delays in the care pathway.
In addition to obvious psychological distress, delay in managing lung cancer increases the potential for disease progression before treatment and may reduce the capacity for treatment with curative intent.3 Brocken and colleagues categorised delay as either “first-line”, caused by delays in the patient seeking medical advice or a delay in management by the general practitioner, or “second-line”, caused by delays in referral (time lag between the hospital receiving a referral and accessing a specialist) and treatment delivery (time lag between diagnosis and the start of treatment).4
Systematic reviews have identified organisational factors that affect the timeliness of care, including whether surgery was undertaken in a teaching hospital, whether the patient was initially referred to someone other than a respiratory physician, and the increasing number of diagnostic tests and hospitals attended to achieve a diagnosis.5 Patient-level factors associated with second-line delays include presentation with atypical symptoms,6 fewer years of education, lower disposable income, and multiple comorbidities,7 as well as symptoms that suggest less advanced disease.8 The reported impact of the age of the patient on timeliness of care is variable.5
In this article we examine second-line delays in the management of NSCLC in Victorian hospitals.
Materials and methods
Patients
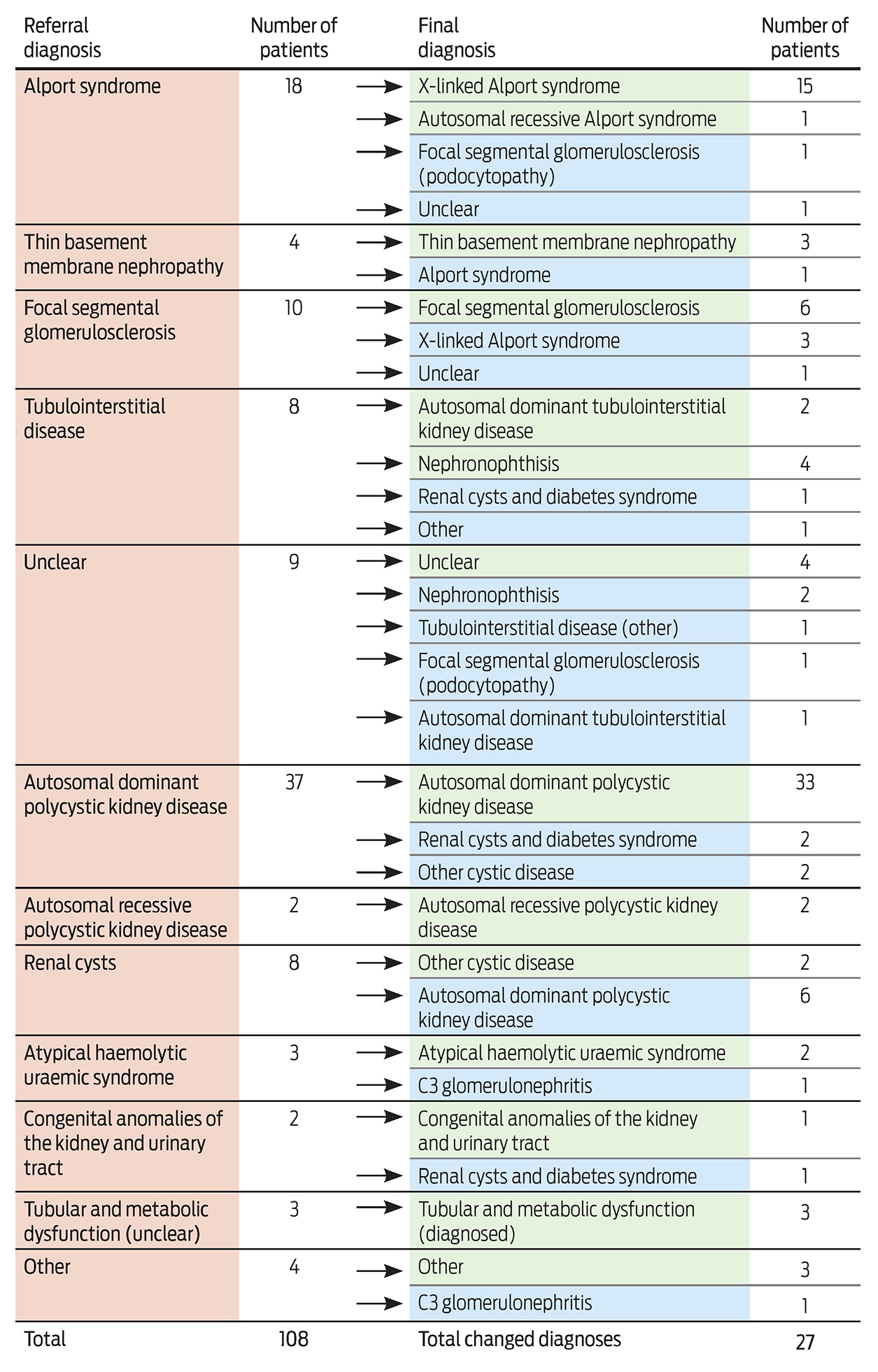
Data were sourced from the Victorian Lung Cancer Registry (VLCR). This initiative operates in eight Victorian hospitals (six public hospitals, including four metropolitan and two regional centres, and two private hospitals) and captures about 25% of Victorian lung cancer notifications.9 Patients were recruited to the registry if they were at least 18 years old and presented with an incident case of lung cancer identified by ICD-10 (International Classification of Diseases, 10th revision) lung cancer codes (C34.0–C34.9, Z85.1, Z85.2) based on either a clinical or pathology diagnosis. Patients were excluded if they had secondary lung cancer or mesothelioma.
Patients diagnosed with NSCLC between July 2011 and October 2014 were assessed for eligibility. A waiver of consent enabled details about deceased patients to be collected. Of the eligible 1863 patients, 446 (24%) were excluded: 267 (14.3%) had been diagnosed by doctors who had not consented to participation in the registry, 67 (3.6%) had a carcinoid tumour, 14 (0.7%) had mesothelioma, and 98 (5.3%) declined participation.
Data collection
Hospital lung cancer notifications were provided by participating hospitals each month. Medical record review was undertaken 4 months after diagnosis. Confirmation of management was obtained when registry staff contacted patients by telephone 6 and 12 months after diagnosis. The medical records of patients who died after diagnosis but before follow-up were used as the only source of information for these patients.
Statistical considerations
Categorical data are presented as absolute numbers and percentages. Continuous variables are presented as means and standard deviations for normally distributed data and medians and interquartile ranges (IQR) for non-parametric data. Time intervals were recorded as medians, IQRs and means.
Patient factors that were analysed included sex, age, country of birth, preferred language, smoking status, TNM stage of disease at diagnosis,10 Eastern Cooperative Oncology Group (ECOG) performance status,11 and major comorbidities extracted from their medical records. Age groups were categorised by quartiles. Patients with diabetes mellitus were defined as those with insulin-dependent or oral hypoglycaemic disease; patients with renal disease were defined as those requiring dialysis; patients with cardiovascular disease were defined as those with a previous myocardial infarction or coronary intervention; patients with respiratory disease were defined as those with a functional expiratory volume of less than 66%; and patients with neoplastic disease were defined as those with any past history of cancer other than lung cancer. The Colinet simplified comorbidity score (SCS) is a weighted index with a range of 0 (no comorbidities) to 20.12 The index was dichotomised into two categories (> 9 v ≤ 9), in line with evidence that an SCS greater than 9 predicts worse survival for patients with NSCLC.13 The Index of Relative Socio-economic Advantage and Disadvantage (IRSAD), which rates the socio-economic status of the patient on the basis of their residential postcode, was categorised into deciles; a low score indicates relatively greater disadvantage and a lack of advantage in general.14
Disease management factors included in our analysis included whether the patient received surgery, radiotherapy or chemotherapy as their initial treatment, and whether the intent was curative or palliative. Organisational factors included hospital type (private v public) and location (metropolitan v regional) for the hospital where diagnosis and initial definitive management were provided.
Three main outcomes were investigated:
-
the interval between initial referral for management and diagnosis (“referral to diagnosis”);
-
the interval between diagnosis and initial surgery, chemotherapy, radiotherapy or referral to palliative care (“diagnosis to initial definitive management”); and
-
the interval between referral and initial definitive management.
The referral date was the date recorded on the referral letter to the hospital or diagnosing clinician. Survival was censored at the date of the event of interest or the date on which the patient died; otherwise, the last known follow-up date (30 December 2014) was used. The log-rank test was used to compare survival in subgroups.
Both univariate and separate multivariate Cox regression analyses were performed to identify independent and significant factors associated with each time interval. For the multivariate model, we started with all significant variables identified in the univariate analysis and applied the stepwise method to determine the final list of variables included in the multivariate model. The likelihood ratio test was performed, with the probability of entry and removal of the variables set at 0.01 and 0.05 respectively. The median time to event was computed from the survival curve (along with the IQR). The proportional hazards assumption was tested using the Schoenfeld test. Data analysis was performed in Stata 13.0 (StataCorp).
Ethics approval
Ethics approval was provided by the Monash University Human Research Ethics Committee (reference CF11/1693–2011000940).
Results
The demographic and clinical characteristics of the 1417 patients included in our analysis are summarised in Box 1. The mean age of the cohort was 71.3 ± 11.4 years. The mean SCS was 7.6 ± 2.9. There were 682 deaths in the cohort (48%).
The median interval between referral and diagnosis was 15 days (IQR, 5–36 days), between diagnosis and initial definitive management 30 days (IQR, 6–84 days), and between referral and definitive management 53 days (IQR, 25–106 days) (Box 2). Socio-demographic factors associated with the length of the intervals between referral and diagnosis, diagnosis and initial definitive management, and referral and initial definitive management are summarised in Box 2. Box 3 includes the clinical factors associated with more timely diagnosis after referral. Hospital-related factors are summarised in Box 4.
Box 5 summarises the results of the stepwise selection process, and details the factors associated with overall delay for each of the three time intervals. The proportional hazards assumption was not violated in the final multivariate model, except for the interval between diagnosis and first management (P < 0.001). This violation was largely associated with the wider differences in the survival slope during the early periods of follow-up for the surgery and no-surgery groups, but, as the plots did not cross at any time point, we decided to retain this variable in the model because it was deemed a clinically important factor.
A longer interval between referral and diagnosis was associated with being born overseas; having early stage disease (stage I or II) or not having the stage documented; notification by a public hospital; receiving curative treatment; and either declining or not receiving palliative care. Patients waited significantly longer for initial definitive management after diagnosis if their ECOG performance status was not documented; having stage II or III disease, or the disease stage at diagnosis was not documented; receiving subsequent treatment in a public hospital; and not undergoing surgery. Overall, a longer interval between referral and initial definitive management was associated with being managed in a public hospital and not receiving (or declining) palliative care.
Discussion
Using a clinical quality registry, we reviewed care provided to a large cohort of patients with NSCLC who were managed in Victorian hospitals. We found several disparities. Patients born overseas experienced delays in receiving a diagnosis after referral, but not in treatment after diagnosis; overall, their treatment path took longer than that of Australian-born patients. Patients with advanced disease received prompt diagnosis, but then experienced delays in further definitive management. This may be explained by patients only needing palliative care after the diagnosis was confirmed. Conversely, patients with early stage disease waited longer to receive a diagnosis but, once diagnosed, received more timely initial definitive management than patients with more advanced disease. Patients managed in public hospitals waited longer than patients managed in the private sector to receive either a diagnosis or initial definitive management. Patients receiving chemotherapy in regional hospitals waited longer than those in metropolitan hospitals. Any active treatment took longer to commence than palliation.
The association between timeliness of care delivery and health outcomes has been investigated in many studies, often with paradoxical results.15 This is partly explained by evidence that patients with more advanced disease are often fast-tracked for referral and definitive management, but are less likely to survive than patients with less advanced disease. Of concern is the accumulating evidence that delay in treatment allows time for tumour growth and may result in patients becoming ineligible for curative treatment. Upstaging rates of 17% within a period of 20 days16 and gross tumour volume increases of 35% over a median time of 13 days17 have been reported.
A systematic review published in 2009 reported that all eight studies which had examined time from referral to first respiratory specialist visit had found median waiting times of no more than 14 days.5 The only published Australian study identified a median time between referral and initial consultation of 5 days, and a further 14 days between initial consultation and a management decision.18 In contrast, median waiting times in our study were as high as 34 days for patients with early stage disease, and patients referred to a metropolitan hospital in which they subsequently underwent surgery waited a median 29 days after referral for a diagnosis.
The time between diagnosis and initiation of treatment for patients with lung cancer has been studied in a number of countries, and the median was found to range between 12 and 52 days.5 Large cohort studies in Canada have identified waiting times of 42 days for curative radiotherapy and 39 days for surgery. Closer to home, the median overall waiting time from diagnosis to initiation of radiation therapy in Queensland was 33 days; this was not affected by distance to the treating hospital.19 Median waiting times were longer for patients with early stage disease (stage I and II NSCLC; median, 48 days) than for those with more advanced disease (stages III and IV disease; median, 34 and 26 days respectively). Our finding that the median waiting time from diagnosis to initiation of radiotherapy in both private and public hospitals was 28 days is comparable with these Queensland findings. However, when our analysis was confined to waiting times in public hospitals, the median waiting time was 30 days, compared with 12 days for patients treated in private facilities.
Dutch quality indicators suggest that organisations should provide a diagnosis within 21 days, and commence treatment within 35 days of the first visit to a specialist.20 The Danish Lung Cancer Registry has established targets of 28 days for the period from referral to diagnosis, 14 days from diagnosis to initial treatment, and 42 days from referral to initial treatment.21 Current standards set by the British National Health Service (NHS) require that all patients with suspected cancer be seen by a specialist within 14 days, and that those diagnosed with cancer be treated within 31 days of the decision to treat and within 62 days of referral.22 While setting a generic waiting time for all cancer management has been criticised as not considering the different levels of priority for the treatment of the various cancer types,23 such targets acknowledge the psychological impact of delay on patients, regardless of the prognosis of the cancer. A study conducted in two Australian radiotherapy centres found that delay between a decision to give radiotherapy and starting treatment (ie, between diagnosis and treatment) caused a higher level of concern than a delay between referral and diagnosis.24
Our finding that patients with less advanced disease waited longer for a diagnosis than patients with advanced disease is consistent with a United Kingdom study of general practitioner referral patterns which showed that a greater proportion of urgent than of non-urgent referrals involved patients with advanced lung cancer disease (higher TNM stage, and extensive v limited stage).25 However, we found that the interval between diagnosis and treatment was shorter for patients with early stage disease (stage I), suggesting that they were given priority for treatment over patients with stage II or III disease.
Our finding that overseas-born patients waited, on average, 5 days longer than their Australian-born counterparts for a diagnosis after referral may reflect organisational (eg, access to translators and services) or patient-level barriers (eg, cultural and health literacy). Cultural barriers explained a 30% reduction in African Americans receiving cancer stage-appropriate treatment in four United States hospitals; African Americans were more likely than their white counterparts to report fatalism, negative surgical beliefs and mistrust of doctors.26 Further work is required to unravel the reasons for the delays experienced by overseas-born patients presenting with suspected lung cancer in Australia.
The finding that patients managed in public hospitals waited more than twice as long as those treated in private hospitals for diagnosis and treatment is disappointing, Particularly unsatisfactory was that the median time from referral to initiation of definitive treatment was 61 days in public hospitals, only just within the British NHS target of a maximum 62 days between referral and first definitive treatment of cancer patients.22 The overall proportion of patients who waited longer than 62 days in our study was 42%, but for patients treated in public hospitals it was 48%.
There were several limitations to our study. First, 20% of patients were excluded from analysis because either the patient or their treating doctor declined participation in the registry. We cannot ascertain whether there were systematic differences in disease management patterns of these patients. Second, caution should be exercised when extrapolating our findings to Victorian hospitals that do not contribute to the VLCR. Patients notified from regional hospitals are under-represented in the registry (15% of cases in our registry were notified from regional Victorian hospitals, while 33% of lung cancer notifications in Victoria over the past 5 years were by regional hospitals27). Finally, the IRSAD score provides socio-economic characteristics of areas in which patients lived at the time of their diagnosis; we were unable to investigate the impact of an individual’s socio-economic status on the timeliness of care.
Improving timeliness of care requires political will and investment in resources to redesign care processes. Interventions that improve timeliness and appropriateness of care, including rapid access lung cancer clinics28 and process restructuring that enables rapid diagnostic imaging, biopsy collection and progression to discussion at multidisciplinary team meetings29 have produced impressive results, and warrant investigation in Victorian public hospitals. Any intervention should be underpinned by systematic monitoring of its impact on the quality of care and feedback to clinical units, such as that provided by the VLCR.
Box 1 –
Demographic and clinical characteristics of the 1417 patients
|
Characteristics
|
Categories
|
Number of patients (percentage)
|
|
|
Age (mean, 71.3 ± 11.4 years)
|
≤ 64 years
|
369 (26.0%)
|
|
65–72 years
|
370 (26.1%)
|
|
73–80 years
|
358 (25.3%)
|
|
≥ 81 years
|
320 (22.6%)
|
|
Sex
|
Male
|
832 (58.7%)
|
|
Born overseas
|
|
719 (50.7%)
|
|
Preference for language other than English
|
|
86 (6.1%)
|
|
Ever smoked
|
|
1139 (90%)
|
|
Notifying hospital type
|
Metropolitan
|
1215 (85.7%)
|
|
Regional
|
202 (14.3%)
|
|
Private
|
428 (30.2%)
|
|
Public
|
989 (69.8%)
|
|
Comorbidities (ascertained from medical record review)
|
Diabetes mellitus
|
195 (13.8%)
|
|
Renal disease
|
24 (1.7%)
|
|
Cardiovascular disease
|
244 (17.2%)
|
|
Respiratory disease
|
206 (14.5%)
|
|
Neoplastic disease
|
292 (20.6%)
|
|
SCS (mean, 7.6 ± 2.9)
|
> 9
|
550 (38.8%)
|
|
ECOG score
|
< 2
|
528 (37.3%)
|
|
2–4
|
194 (13.7%)
|
|
Not available/not stated
|
695 (49.1%)
|
|
Disease stage at diagnosis*
|
I
|
122 (8.6%)
|
|
II
|
135 (9.5%)
|
|
III
|
201 (14.2%)
|
|
IV
|
253 (17.9%)
|
|
Not available/not stated
|
706 (49.8%)
|
|
IRSAD, percentiles (mean score: 1020 [SD, 73])
|
1%–20%
|
206 (14.6%)
|
|
21%–40%
|
184 (13.0%)
|
|
41%–60%
|
246 (17.4%)
|
|
61%–80%
|
276 (19.5%)
|
|
81%–100%
|
502 (35.5%)
|
|
First treatment intent was curative
|
|
461 (44.2%)
|
|
Surgery performed
|
|
459 (32.4%)
|
|
Chemotherapy performed
|
|
548 (38.7%)
|
|
Radiotherapy performed
|
|
487 (34.4%)
|
|
Palliative care
|
Referral
|
404 (28.5%)
|
|
Not performed/declined
|
835 (58.9%)
|
|
Not stated
|
178 (12.6%)
|
|
Treating hospital for surgery
|
Metropolitan
|
437 (95.2%)
|
|
Regional
|
22 (4.8%)
|
|
Private
|
179 (39.0%)
|
|
Public
|
280 (61.0%)
|
|
Treating hospital for chemotherapy
|
Metropolitan
|
406 (75.9%)
|
|
Regional
|
129 (24.1%)
|
|
Private
|
177 (33.1%)
|
|
Public
|
358 (66.9%)
|
|
Treating hospital for radiotherapy
|
Metropolitan
|
367 (77.4%)
|
|
Regional
|
107 (22.6%)
|
|
Private
|
71 (15.0%)
|
|
Public
|
403 (85.0%)
|
|
|
ECOG = Eastern Cooperative Oncology Group11; IRSAD = Index of Relative Socio-economic Advantage and Disadvantage14; SCS = Colinet simplified comorbidity score (SCS)12; SD = standard deviation. * TNM classification of malignant tumours (7th edition).10
|
Box 2 –
Socio-demographic factors associated with length of the time intervals (in days) between referral and diagnosis, diagnosis and first management, and referral and first definitive management
|
Characteristic
|
Referral to diagnosis
|
Diagnosis to initial definitive management
|
Referral to initial definitive management
|
|
Median (IQR)
|
P*
|
Median (IQR)
|
P*
|
Median (IQR)
|
P*
|
|
|
Overall
|
15 (5–36)
|
|
30 (6–84)
|
|
53 (25–106)
|
|
|
Age group
|
|
0.133
|
|
< 0.001
|
|
< 0.001
|
|
≤ 64 years
|
14 (5–32)
|
|
24 (5–60)
|
|
43 (20–81)
|
|
|
65–72 years
|
16 (5–41)
|
|
25 (0–66)
|
|
47 (23–100)
|
|
|
73–80 years
|
16 (6–40)
|
|
31 (3–75)
|
|
56 (32–112)
|
|
|
≥ 81 years
|
13 (4–31)
|
|
46 (14–NC)
|
|
68 (31–421)
|
|
|
Sex
|
|
|
|
|
|
|
|
Female
|
13 (5–34)
|
0.498
|
31 (5–97)
|
0.271
|
52 (24–120)
|
0.282
|
|
Male
|
16 (5–37)
|
|
30 (7–75)
|
|
53 (25–102)
|
|
|
Place of birth
|
|
|
|
|
|
|
|
Australia
|
13 (4–33)
|
0.001
|
26 (1–82)
|
0.063
|
46 (20–102)
|
0.019
|
|
Overseas
|
18 (6–40)
|
|
33 (10–88)
|
|
57 (31–112)
|
|
|
Language preference
|
|
|
|
|
|
|
|
English
|
15 (5–35)
|
0.031
|
30 (6–84)
|
0.491
|
51 (23–105)
|
0.031
|
|
Other language
|
20 (7–61)
|
|
36 (14–72)
|
|
70 (42–131)
|
|
|
Smoking history
|
|
|
|
|
|
|
|
Never smoked
|
12 (4–31)
|
0.398
|
27 (1–59)
|
0.456
|
43 (17–87)
|
0.175
|
|
Ever smoked
|
16 (6–38)
|
|
28 (4–70)
|
|
53 (26–105)
|
|
|
IRSAD deciles
|
|
0.533
|
|
0.089
|
|
0.068
|
|
1
|
19 (7–40)
|
|
34 (7–80)
|
|
56 (29–105)
|
|
|
2
|
27 (11–44)
|
|
50 (4–434)
|
|
73 (34–261)
|
|
|
3
|
15 (8–34)
|
|
26 (0–63)
|
|
52 (3–96)
|
|
|
4
|
14 (6–40)
|
|
51 (7–731)
|
|
62 (33–147)
|
|
|
5
|
17 (6–45)
|
|
29 (1–88)
|
|
61 (20–111)
|
|
|
6
|
14 (5–35)
|
|
28 (3–88)
|
|
43 (24–100)
|
|
|
7
|
16 (7–32)
|
|
29 (4–69)
|
|
59 (26–98)
|
|
|
8
|
13 (4–37)
|
|
28 (6–59)
|
|
43 (21–89)
|
|
|
9
|
13 (3–39)
|
|
29 (9–90)
|
|
59 (26–118)
|
|
|
10
|
11 (3–25)
|
|
25 (8–63)
|
|
43 (19–94)
|
|
|
|
IRSAD = Index of Relative Socio-economic Advantage and Disadvantage; NC = not computable. * Log-rank test comparison across each category for the respective variable.
|
Box 3 –
Clinical factors associated with length of time intervals (in days) between referral to diagnosis, diagnosis to first management, and referral to first definitive management
|
Characteristic
|
Referral and diagnosis
|
Diagnosis and initial definitive management
|
Referral and initial definitive management
|
|
Median (IQR)
|
P*
|
Median (IQR)
|
P*
|
Median (IQR)
|
P*
|
|
|
Comorbidities†
|
|
|
|
|
|
|
|
Diabetes mellitus present
|
18 (5–44)
|
0.395
|
29 (3–58)
|
0.048
|
55 (31–91)
|
0.635
|
|
Diabetes mellitus absent
|
14 (5–35)
|
|
30 (6–90)
|
|
52 (24–108)
|
|
|
Renal disease present
|
15 (2–40)
|
0.746
|
31 (19–49)
|
0.876
|
49 (32–182)
|
0.679
|
|
Renal disease absent
|
15 (5–36)
|
|
30 (6–84)
|
|
53 (24–106)
|
|
|
Cardiovascular disease present
|
20 (6–50)
|
0.024
|
25 (4–59)
|
0.023
|
55 (27–106)
|
0.910
|
|
Cardiovascular disease absent
|
14 (5–34)
|
|
31 (6–88)
|
|
52 (24–106)
|
|
|
Respiratory disease present
|
25 (10–51)
|
< 0.001
|
40 (12–85)
|
0.121
|
69 (40–129)
|
< 0.001
|
|
Respiratory disease absent
|
14 (4–33)
|
|
28 (6–84)
|
|
50 (22–102)
|
|
|
Neoplastic disease present
|
16 (5–40)
|
0.156
|
30 (4–77)
|
0.516
|
55 (24–128)
|
0.653
|
|
Neoplastic disease absent
|
15 (5–36)
|
|
30 (6–85)
|
|
52 (25–104)
|
|
|
SCS < 9
|
15 (6–36)
|
0.345
|
30 (4–77)
|
0.281
|
54 (26–111)
|
0.191
|
|
SCS ≥ 9
|
15 (4–36)
|
|
30 (8–94)
|
|
50 (22–102)
|
|
|
ECOG score < 2
|
14 (5–34)
|
< 0.001
|
24 (1–51)
|
< 0.001
|
47 (22–87)
|
< 0.001
|
|
ECOG score 2–4
|
9 (3–22)
|
|
40 (12–140)
|
|
56 (24–244)
|
|
|
ECOG score missing
|
18 (6–44)
|
|
37 (2–218)
|
|
59 (29–129)
|
|
|
Disease stage at diagnosis‡
|
|
< 0.001
|
|
< 0.001
|
|
0.078
|
|
I
|
34 (16–61)
|
|
0 (0–37)
|
|
56 (33–103)
|
|
|
II
|
29 (11–60)
|
|
28 (0–58)
|
|
59 (33–102)
|
|
|
III
|
17 (7–28)
|
|
38 (15–64)
|
|
55 (34–83)
|
|
|
IV
|
10 (4–23)
|
|
30 (12–72)
|
|
44 (22–91)
|
|
|
Not available/not stated
|
13 (4–34)
|
|
32 (7–339)
|
|
54 (20–161)
|
|
|
First treatment intent non-curative
|
12 (4–24)
|
< 0.001
|
11 (25–47)
|
< 0.001
|
37 (19–63)
|
0.017
|
|
First treatment intent curative
|
28 (11–58)
|
|
0 (0–31)
|
|
45 (22–78)
|
|
|
Surgery performed
|
28 (10–56)
|
< 0.001
|
0 (0–22)
|
< 0.001
|
42 (18–73)
|
< 0.001
|
|
Surgery not performed
|
11 (4–25)
|
|
48 (21–393)
|
|
61 (30–230)
|
|
|
Chemotherapy performed
|
13 (4–30)
|
< 0.001
|
24 (7–47)
|
< 0.001
|
38 (19–68)
|
< 0.001
|
|
Chemotherapy not performed
|
16 (6–42)
|
|
41 (4–NC)
|
|
69 (32–414)
|
|
|
Radiotherapy performed
|
13 (4–25)
|
< 0.001
|
28 (11–52)
|
< 0.001
|
42 (21–73)
|
< 0.001
|
|
Radiotherapy not performed
|
17 (5–43)
|
|
32 (0–739)
|
|
63 (30–181)
|
|
|
Palliative care referral
|
11 (3–22)
|
< 0.001
|
43 (19–255)
|
< 0.001
|
56 (26–175)
|
0.007
|
|
Palliative care not performed
|
19 (7–46)
|
|
23 (0–55)
|
|
51 (27–97)
|
|
|
Not stated
|
8 (2–24)
|
|
54 (10–NC)
|
|
44 (17–NC)
|
|
|
|
ECOG = Eastern Cooperative Oncology group11; IRSAD = Index of Relative Socio-economic Advantage and Disadvantage14; NC = not computable; SCS = Colinet simplified comorbidity score (SCS).12 * Log-rank test comparison across each category for the respective variable. The difference associated with ECOG scores (< 2 v 2–4) was also significant if the not available/not stated group was excluded (P < 0.001). The comparisons for comorbidities are between those who had the comorbidity versus those who did not. † Comorbidities ascertained from medical record review. ‡ TNM classification of malignant tumours (7th edition).10
|
Box 4 –
Hospital-related factors associated with length of time intervals (in days) between referral and diagnosis, diagnosis and first management, and referral and first definitive management
|
Characteristic
|
Referral to diagnosis
|
Diagnosis to initial definitive management
|
Referral to initial definitive management
|
|
Median (IQR)
|
P*
|
Median (IQR)
|
P*
|
Median (IQR)
|
P*
|
|
|
Notifying hospital
|
|
|
|
|
|
|
|
Metropolitan hospital
|
15 (5–38)
|
0.289
|
NA
|
|
NA
|
|
|
Regional hospital
|
18 (10–29)
|
|
NA
|
|
NA
|
|
|
Private hospital
|
7 (2–19)
|
< 0.001
|
NA
|
|
NA
|
|
|
Public hospital
|
19 (8–43)
|
|
NA
|
|
NA
|
|
|
Treating hospital
|
|
|
|
|
|
|
|
Private
|
NA
|
|
15 (1–52)
|
|
30 (13–76)
|
|
|
Public
|
NA
|
|
36 (11–91)
|
< 0.001
|
61 (35–118)
|
< 0.001
|
|
Treating hospital for surgery
|
|
|
|
|
|
|
|
Metropolitan
|
29 (9–59)
|
0.250
|
0 (0–20)
|
0.065
|
41 (18–73)
|
0.741
|
|
Regional
|
23 (16–44)
|
|
21 (0–58)
|
|
44 (34–72)
|
|
|
Private
|
15 (5–38)
|
< 0.001
|
0 (0–16)
|
0.040
|
22 (10–46)
|
< 0.001
|
|
Public
|
35 (15–70)
|
|
0 (0–28)
|
|
51 (30–91)
|
|
|
Treating hospital for chemotherapy
|
|
|
|
|
|
|
|
Metropolitan
|
11 (3–90)
|
0.010
|
20 (6–40)
|
0.001
|
37 (17–65)
|
0.068
|
|
Regional
|
20 (10–31)
|
|
32 (11–58)
|
|
43 (28–79)
|
|
|
Private
|
5 (1–16)
|
< 0.001
|
14 (4–30)
|
< 0.001
|
20 (11–38)
|
< 0.001
|
|
Public
|
19 (8–37)
|
|
29 (9–51)
|
|
50 (30–78)
|
|
|
Treating hospital for radiotherapy
|
|
|
|
|
|
|
|
Metropolitan
|
12 (4–25)
|
0.964
|
26 (10–49)
|
0.099
|
41 (20–70)
|
0.515
|
|
Regional
|
16 (6–26)
|
|
35 (15–61)
|
|
50 (23–78)
|
|
|
Private
|
3 (1–8)
|
< 0.001
|
12 (6–31)
|
0.005
|
20 (12–43)
|
< 0.001
|
|
Public
|
15 (7–29)
|
|
30 (13–54)
|
|
46 (25–76)
|
|
|
|
NA = not available. * Log-rank test comparison across each category for the respective variable.
|
Box 5 –
Multivariate analysis of factors associated with lengths of intervals
|
Characteristics
|
Hazard ratio (95% CI)*
|
P†
|
|
|
Characteristics affecting time from referral to diagnosis
|
|
Place of birth
|
|
|
|
Australia
|
1
|
|
|
Overseas
|
0.84 (0.72–0.99)
|
0.035
|
|
Disease stage at diagnosis‡
|
|
|
|
I
|
0.58 (0.43–0.78)
|
0.000
|
|
II
|
0.66 (0.49–0.89)
|
0.006
|
|
III
|
0.92 (0.72–1.18)
|
0.529
|
|
IV
|
1
|
|
|
Not available/not stated
|
0.74 (0.59–0.93)
|
0.010
|
|
Notifying hospital
|
|
|
|
Private
|
1
|
|
|
Public
|
0.50 (0.41–0.60)
|
< 0.001
|
|
First treatment intent
|
|
|
|
Non-curative
|
1
|
|
|
Curative
|
0.73 (0.61–0.89)
|
0.002
|
|
Palliative Care
|
|
|
|
Yes
|
1
|
|
|
No/declined
|
0.64 (0.52–0.79)
|
< 0.001
|
|
Not stated
|
1.22 (0.87–1.71)
|
0.245
|
|
Factors affecting time from diagnosis to initial definitive management
|
|
ECOG performance status
|
|
|
|
< 2
|
1
|
|
|
2–4
|
0.95 (0.77–1.16)
|
0.615
|
|
Not available/not stated
|
0.84 (0.74–0.96)
|
0.011
|
|
Disease stage at diagnosis†
|
|
|
|
I
|
1
|
|
|
II
|
0.60 (0.46–0.79)
|
< 0.001
|
|
III
|
0.61 (0.47–0.78)
|
< 0.001
|
|
IV
|
0.81 (0.63–1.05)
|
0.118
|
|
Not available/not stated
|
0.79 (0.63–0.99)
|
0.044
|
|
Treating hospital type
|
|
|
|
Private
|
1
|
|
|
Public
|
0.80 (0.69–0.92)
|
0.002
|
|
Surgery performed
|
|
|
|
Yes
|
1
|
|
|
No/declined
|
0.54 (0.47–0.62)
|
< 0.001
|
|
Factors affecting time from referral to initial definitive management
|
|
Palliative care
|
|
|
|
Yes
|
1
|
|
|
No/declined
|
0.73 (0.62–0.86)
|
< 0.001
|
|
Not stated
|
1.03 (0.78–1.35)
|
0.849
|
|
Treating hospital type
|
|
|
|
Private
|
1
|
|
|
Public
|
0.55 (0.48–0.64)
|
< 0.001
|
|
|
ECOG = Eastern Cooperative Oncology group. * A positive hazard ratio corresponds to shorter time to event. † Cox proportional hazards model. ‡ TNM classification of malignant tumours (7th edition).10
|

 more_vert
more_vert