The known Early mobilisation and daily physiotherapy after hip fracture fixation are recommended by guidelines, but there is no evidence that guides the intensity of acute hospital physiotherapy for this patient population.
The new Intensive acute hospital physiotherapy following an isolated hip fracture reduced hospital length of stay by more than 10 days without increasing complication or re-admission rates.
The implications We have provided evidence-based support for intensive physiotherapy programs in the acute hospital setting after hip fracture. Our findings may have significant practical implications, given the large number of inpatient beds occupied by this patient group.
Rising rates of hip fracture in our ageing population and the consequently increasing costs of care are significant problems for the hospital system and the community.1 In Australia, 17 000 hip fractures in people aged 65 years or more incur direct hospital costs of $579 million each year,2 and it is projected that the annual number of hip fractures will rise to 60 000 by 2051.1 Costs continue to accrue after patients leave hospital, with about 25% requiring full-time nursing home care3,4 and 50% of previously independent patients needing a gait aid or long term help with routine activities.3
Many strategies have been tried and guidelines developed for optimising the management of this population, including reducing the time to surgery and improving analgesia.5 Several rehabilitation strategies have also been investigated, including early assisted ambulation (within 48 hours of surgery)6 and multidisciplinary programs.7
There are no recommendations about the intensity of physiotherapy during the acute post-operative phase following hip fracture fixation. One cohort study found that increased intensity of acute hospital physiotherapy was associated with a greater likelihood of discharge home.8 Intensive physiotherapy for trauma patients was recently found to be safe, leading to more rapid improvements in mobility.9 Whether these findings can be extrapolated to patients with fractured hips or if early, intensive intervention has longer term benefits are, however, unknown.
The aim of our study was to investigate the effect of providing an intensive physiotherapy program for patients aged 65 years or more with isolated hip fractures.
Methods
Design and ethics approval
This was a single-centre, prospective randomised controlled trial at The Alfred, a level 1 trauma centre in Melbourne. Approval was granted by the Alfred Health Human Research and Ethics Committee (reference, 32/14). The study was registered with the Clinical Trials Registry in March 2014 (https://clinicaltrials.gov/ct2/show/NCT02088437).
Subjects
Between March 2014 and January 2015, all patients admitted to The Alfred with isolated hip fractures were screened for study inclusion. If patients were unable to provide consent, it was requested from the person responsible for their medical decisions. Patients were included if they were at least 65 years old, had been admitted with an isolated subcapital or intertrochanteric hip fracture, and were treated by internal fixation or hemiarthroplasty. Patients were excluded if the fracture was subtrochanteric or pathological, and if post-operative orders required the patient to be non-weight-bearing on the operated hip. Patients who were unable to move independently or who needed a gait aid prior to admission, and those admitted from nursing homes were also excluded.
Participants were randomly assigned by computer program to one of two groups: usual care (the control group), in which they received one daily treatment session of 30 minutes; or intensive physiotherapy (the intervention group), in which they received two additional daily treatment sessions of about 30 minutes each. Allocation was concealed by using opaque envelopes.
Physiotherapy
The usual care physiotherapist was blinded to the allocation, and treated all patients in the morning. The intervention group physiotherapist and allied health assistant provided physiotherapy during the afternoon; this was not documented in the patient’s medical record, maintaining the blinding of the usual care therapist and treating team.
Usual physiotherapy care
Patients in both groups received daily physiotherapy according to usual practice in the trauma centre, 7 days per week. Treatment was individualised and involved bed-based limb exercises (eg, strength exercises, such as knee extension, and active hip exercises) and gait re-training. The goal was early, independent transfer and mobility, with the objective of discharge directly home or to fast stream rehabilitation.
Intervention group
Patients in the intervention group received two additional daily sessions, 7 days per week. One session, delivered by an allied health assistant, practised the achievements of the morning session. An additional session, delivered by a physiotherapist, aimed to improve the functional advances achieved during the earlier physiotherapy session; eg, increased independence, progression of gait aid (eg, from frame to crutches), and increasing the distance walked.
Discharge criteria
A physician blinded to group allocation reviewed all patients, and determined medical stability and the need for inpatient rehabilitation. Participants were discharged home once they were medically stable, were deemed physically ready to return home by the blinded physiotherapist, and had been cleared by the multidisciplinary team. Physical readiness was defined as independence in bed and chair transfers, walking with a gait aid, and negotiation of any stairs required by the patient to safely enter or leave their home.9,10 If patients were unable to achieve these criteria before medical stability was achieved, they were transferred to an inpatient rehabilitation facility, per hospital protocol.
Data collection
Demographic data collected included sex, age, mechanism of injury, pre-morbid mobility level, social circumstances (residence, support at home) and home set-up (distance and steps to house entrance). Cognitive function was assessed by the physician with the Mini-Mental Status Examination, with scores ranging from 0 to 30 points: patients scoring 26–30 have no functional cognitive impairments, those scoring 20–25 display mild cognitive impairments, and those scoring less than 20 points having moderate to severe cognitive impairments and usually cannot live independently.11 Operative data, including fracture type (subcapital, intertrochanteric), operative time, post-operative weight-bearing status, and American Society of Anesthesiologists (ASA) score,12 were also collected. The ASA score provides a six-category physical status classification system, ranging from the normal healthy person (1) to brain-dead (6).
Outcome data were collected on post-operative Day 5, at discharge, and at 6 months. The primary outcome, the modified Iowa Level of Assistance (mILOA) score, was measured by a blinded assessor on post-operative Day 5 (or on the day of discharge, if this occurred earlier). The ILOA scale was originally designed for assessing patients with lower limb arthroplasty,13 and was later modified for patients who had fractured a hip.6 The mILOA is a functional score with six domains, including bed and chair transfer, ambulation, ascending/descending one step, and gait aid used. The score ranges from 0 (independent in all activities without a gait aid) to 36 (unable to attempt any of the activities). The scale has been validated in an acute hospital population14 and was found to be responsive in an earlier physiotherapy trial.9 Secondary outcome measures included a Timed Up and Go (TUG) test,15 administered by a blinded assessor to patients who were mobilising without assistance at Day 5 (a prerequisite for this test). Data on acute hospital length of stay (LOS), inpatient rehabilitation LOS and combined hospital LOS, inpatient complications, and re-admissions were also collected. Time to physical readiness for discharge, based on the criteria for discharge home applied by an elective joint replacement early discharge program,10 was also recorded. Other data included discharge destination from the acute hospital (home; slow or fast stream rehabilitation) and pain scores before and after physiotherapy. The amount of pain relief medication taken during the first 72 hours after surgery was recorded as the opioid equivalence score.16 Six-month outcome information was derived from routinely collected data in the Victorian Orthopaedic Trauma Outcomes Registry (VOTOR), including discharge destination, and Short Form 12 Health Survey (SF-12), Glasgow Outcome Scale (extended) (GOS-E), and EuroQol five dimensions health state questionnaire (EQ-5D) scores.17 When the patient was unable to provide a response to the EQ-5D, proxy responses were substituted.18
Statistical analysis
Ninety-two patients were required to detect a genuine difference of seven units on the mILOA scale at Day 5 (80% power, α = 0.05 [two-sided]),13 assuming that the standard deviation of the response variable was 11 (based on data reported for this outcome in a previous study19). This sample size calculation allowed for an anticipated drop-out rate of 15%.
Analyses were performed in Stata 12.0 (StataCorp). Data are presented as means and standard deviations, or as medians and interquartile ranges if the data were not normally distributed. Differences between groups at baseline were assessed with independent samples t tests (continuous data) or χ2 tests (categorical data). Data for the outcomes LOS, time to walk, time to sit out of bed, sum of hours, occasions of service, and physical readiness for discharge were not normally distributed, so these variables were natural log-transformed. Differences in outcome variables between treatment groups were compared by univariate analysis of variance (continuous variables) or with χ2 tests (categorical variables). The GOS-E data were dichotomised into scores of ≥ 7 (“good recovery”) or < 7 (“less than good recovery”)20 and analysed in χ2 tests. mILOA scores and combined LOS were assessed by linear regression; all potential confounders related to these outcomes in the univariate analysis (P < 0.2) were included in this analysis.
The effect of group allocation on time to discharge (as measured by combined LOS) was evaluated by Cox proportional hazard regression. Corresponding Kaplan–Meier curves were constructed and compared in log-rank tests.
Results
Between March 2014 and January 2015, 170 patients were screened; 68 were excluded, and 92 patients were recruited (46 allocated to each group). One control group patient was unable to participate in physiotherapy during the first 7 days because of a post-operative complication (dislocation prior to the initial physiotherapy intervention). No patients withdrew from the study, and Day 5 outcome measures were collected for all patients. The trial complied fully with CONSORT guidelines (http://www.consort-statement.org/); the CONSORT flow diagram for participation is included in the Appendix.
Demographic data for the two groups are summarised in Box 1. The groups were well matched for age, fracture type, and length of surgery, as well as for pre-operative mILOA and ASA scores. There was a significant difference between the groups for anaesthetic type, with patients in the intervention group more likely to have had a general anaesthetic. The patients in the intervention group were also less likely to have support at home, and there was a trend towards a greater proportion of women in this group.
There was no difference between the groups in the primary outcome measure, mILOA score on post-operative Day 5, with a mean difference of 2.7 points (mean scores: control group, 19.2 [SD, 8.4]; intervention group, 16.5 [SD, 9.4]; P = 0.10) (Box 2). However, after controlling for potential confounding factors (sex, anaesthetic type, carer at home, stairs at home), the Day 5 mILOA score was lower (better) in the intervention group (P = 0.04, Box 3). Hospital LOS (acute and inpatient rehabilitation) was 10.6 days shorter in the intervention group (median LOS: control, 35.0 days [IQR, 19.0–49.8]; intervention, 24.4 days [IQR, 16.4–31.6]; P = 0.01). This difference persisted in a linear regression that controlled for possible confounding factors (Box 4).
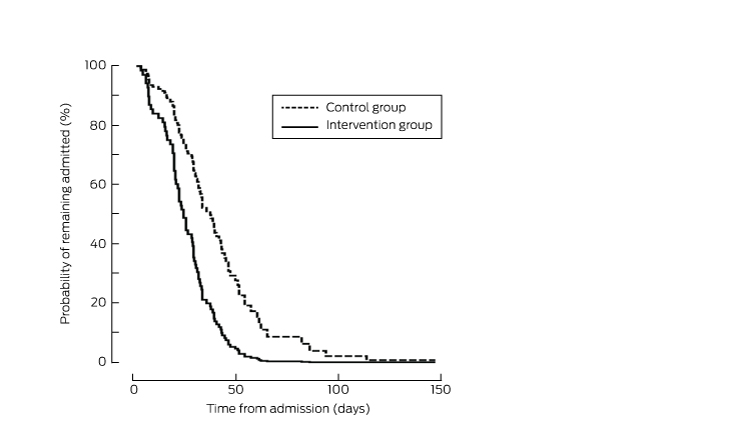
A Cox proportional hazards model that controlled for sex, anaesthetic type, presence of a carer at home, and presence of stairs at home found that the intervention conferred a benefit in terms of earlier discharge from hospital (hazard ratio, 2.30; 95% CI, 1.40–3.78; P < 0.001) (Box 5).
There was no difference between the two trial groups in mean pain or opioid equivalent scores over the first 3 post-operative days (Box 6), nor were there differences in the rates of complications or re-admission, or in longer term patient-reported outcomes (Box 2).
Discussion
We found that an intensive physiotherapy program beginning on the first day after surgery for an isolated hip fracture in patients over 65 years of age is safe and effective. There was no statistically significant difference between control and intervention groups in the primary outcome of functional mobility on post-operative Day 5, but hospital LOS was significantly lower for the intensive physiotherapy group. There were also no differences between groups with respect to pain levels or opioid pain relief requirements. These findings further support the current guidelines, which recommend physiotherapy after a hip fracture to facilitate early assisted ambulation.
After controlling for relevant confounders, our primary outcome, mILOA score, was significantly better in the intervention group at Day 5 (Box 3). Functional mobility at discharge is a major determinant of mortality,21 and whether this improved mobility affects longer term outcomes should be investigated. We powered our study to detect a 7-point difference, as this has been reported as the smallest difference in mILOA score of clinical importance.13 The between-group difference in favour of the intervention group did not reach this threshold, so the difference we found may not be clinically significant. Unfortunately, we only collected data on the primary outcome measure at one post-operative time point; Day 5 may be too early to detect a substantial difference in functional mobility in this patient group, and assessing function at longer time intervals would be desirable.
The economic burden of hip fractures is great;3,22 they account for 14% of all osteoporotic fractures, but for 72% of costs, which are largely associated with inpatient care.22 We found that hospital LOS (a secondary outcome measure) was shorter in the intervention group, with a 10-day reduction across acute and subacute care, reflected by a similar improvement in the time to physical readiness for discharge. This was achieved without an increase in rates of in-hospital complications or re-admissions, and without affecting 6-month outcomes. The difference in LOS is still evident well into the rehabilitation period (Box 5). This may represent an important cost saving, given the large numbers of patients requiring management in hospital systems worldwide, and the high costs associated with their care.
The strengths of our study were its robust design, the randomisation protocols, and the blinding of both the usual care physiotherapist and the outcome assessor to trial group allocation. No patient was lost to follow-up during the in-hospital phase of data collection, and 6-month outcome scores could be collected for more than 85% of participants.
The limitations of our study include the relatively small numbers in each trial group (the result of the single institution trial design) and the short duration of the intervention (limited to acute hospital stay). Although our cohort was randomised, there were minor differences between the groups at baseline; more patients in the intervention group had received a general anaesthetic, and fewer had support at home. These differences highlight the importance of our findings, as previous studies have shown that patients who receive a general anaesthetic have an increased rate of post-operative complications,23 and those without support at home are more likely to require assistance after discharge and to be discharged to a higher level of care.24 Further, most patients were at home prior to admission, limiting the generalisability of our findings. We have not provided a robust cost analysis of the project or detailed information regarding resource allocation, although the financial benefit of reducing hospital LOS is clear.
Conclusion
We found that an intensive physiotherapy program for patients in the acute care setting after an isolated hip fracture can reduce hospital LOS by more than 10 days without increasing the rates of complications or re-admissions. Our study provides support for providing intensive physiotherapy programs for those with hip fractures, and this should be considered in future care guidelines.
Box 1 –
Demographic data for the usual care (control) and intensive physiotherapy (intervention) groups
|
Characteristic
|
Usual care
|
Intensive physiotherapy
|
P
|
|
|
Number of patients
|
46
|
46
|
|
|
Age (years), mean (SD)
|
81.3 (9.0)
|
81.3 (7.5)
|
1.00
|
|
Sex
|
|
|
|
|
Men
|
21 (46%)
|
12 (26%)
|
0.05
|
|
Women
|
25 (54%)
|
34 (74%)
|
|
|
Fracture type
|
|
|
|
|
Subcapital
|
30 (65%)
|
25 (54%)
|
0.29
|
|
Intertrochanteric
|
16 (35%)
|
21 (46%)
|
|
|
Pre-operative modified Iowa Level of Assistance score, mean (SD)
|
1.74 (2.80)
|
1.39 (2.56)
|
0.54
|
|
Pre-operative American Society of Anesthesiologists score
|
|
|
|
|
1
|
4 (9%)
|
1 (2%)
|
0.36
|
|
2
|
10 (22%)
|
15 (33%)
|
|
|
≥ 3
|
32 (70%)
|
30 (65%)
|
|
|
Mini-Mental Status Examination score*
|
|
|
|
|
26–30
|
5 (14%)
|
6 (17%)
|
0.15
|
|
20–25
|
16 (44%)
|
8 (23%)
|
|
|
< 20
|
15 (43%)
|
21 (60%)
|
|
|
Residence
|
|
|
|
|
Home
|
44 (96%)
|
42 (91%)
|
0.24
|
|
Retirement village
|
1 (2%)
|
0
|
|
|
Low level care
|
1 (2%)
|
4 (9%)
|
|
|
Carer at home*
|
|
|
|
|
Yes
|
24 (53%)
|
14 (31%)
|
0.03
|
|
No
|
21 (47%)
|
31 (69%)
|
|
|
Stairs at home
|
|
|
|
|
Yes
|
36 (78%)
|
29 (63%)
|
0.11
|
|
No
|
10 (22%)
|
17 (37%)
|
|
|
Health care funding*
|
|
|
|
|
Private
|
8 (21%)
|
13 (31%)
|
0.36
|
|
Medicare
|
30 (79%)
|
28 (67%)
|
|
|
Transport Accident Commission
|
0
|
1 (2%)
|
|
|
Anaesthetic type
|
|
|
|
|
General
|
34 (74%)
|
42 (91%)
|
0.03
|
|
Spinal
|
12 (26%)
|
4 (9%)
|
|
|
Length of surgery (minutes), median (IQR)
|
82 (62–101)
|
74.5 (55–101)
|
0.96
|
|
|
* Missing data mean that numbers do not add to column total.
|
Box 2 –
Outcomes for the usual care (control) and intensive physiotherapy (intervention) groups
|
Outcome
|
Usual care
|
Intensive physiotherapy
|
P
|
|
|
Number of patients
|
46
|
46
|
|
|
Modified Iowa Level of Assistance (mILOA) score, mean (SD)
|
19.2 (8.4)
|
16.5 (9.4)
|
0.15
|
|
Timed Up and Go conducted
|
16 (35%)
|
24 (52%)
|
0.09
|
|
Timed Up and Go (seconds), median (IQR)
|
69 (51.5–96.5)
|
47 (31–89)
|
0.08
|
|
Acute hospital length of stay (days), median (IQR)
|
7.4 (5.4–8.8)
|
6.0 (4.6–7.2)
|
0.08
|
|
Inpatient rehabilitation length of stay (days), median (IQR)
|
29.9 (15.9–42.9)
|
21.0 (13.8–26.8)
|
0.06
|
|
Combined hospital length of stay (days), median (IQR)
|
35.0 (19.0–49.8)
|
24.4 (16.4–31.6)
|
0.01
|
|
Time to physical readiness for discharge (days), median (IQR)
|
27.7 (14.0–37.6)
|
16.3 (5.8–25.6)
|
0.02
|
|
Physiotherapy occasions of service, median (IQR)
|
5 (3–6)
|
10.5 (7–14)
|
< 0.001
|
|
Time spent in physiotherapy (hours), median (IQR)
|
115 (70–160)
|
248.5 (145–290)
|
< 0.001
|
|
Time to sit on bed (days), median (IQR)
|
1.02 (0.9–1.8)
|
1.0 (0.9–1.5)
|
0.43
|
|
Time to walk 3 metres (days), median (IQR)
|
1.95 (1.0–4.6)
|
1.93 (1.1–3.0)
|
0.19
|
|
Discharge destination from acute hospital
|
|
|
0.31
|
|
Home
|
5 (11%)
|
10 (22%)
|
|
|
Fast stream rehabilitation
|
11 (24%)
|
12 (26%)
|
|
|
Slow stream rehabilitation
|
30 (65%)
|
24 (52%)
|
|
|
Final discharge destination
|
|
|
0.86
|
|
Home
|
33 (72%)
|
36 (78%)
|
|
|
Low level care
|
7 (15%)
|
6 (13%)
|
|
|
High level care
|
6 (13%)
|
4 (9%)
|
|
|
In-hospital complications
|
|
|
|
|
Hip dislocation
|
1 (2%)
|
0
|
0.32
|
|
Troponin levels > 26 ng/L
|
3 (7%)
|
5 (11%)
|
0.46
|
|
Anaemia requiring transfusion
|
9 (20%)
|
11 (24%)
|
0.61
|
|
Acute kidney injury
|
8 (17%)
|
4 (9%)
|
0.22
|
|
Re-admission within 6 months
|
|
|
0.25
|
|
Hip-related
|
4 (9%)
|
2 (4%)
|
|
|
Not hip-related
|
12 (26%)
|
3 (7%)
|
|
|
6-month Glasgow Outcome Scale (extended) (GOS-E) score*
|
|
|
0.10
|
|
< 7
|
26
|
17
|
|
|
≥ 7
|
15
|
21
|
|
|
6-month EuroQol (5 dimensions) (EQ-5D), mean score (SD)†
|
63.1 (22.3)
|
70 (16.7)
|
0.15
|
|
6-month SF-12, mental components, mean score (SD)‡
|
59.3 (3.7)
|
56.8 (7.0)
|
0.27
|
|
6-month SF-12, physical components, mean score (SD)‡
|
44.3 (11.8)
|
41.7 (11.9)
|
0.56
|
|
|
SF-12 = Short Form 12 health survey. * n = 41 (control group), n = 38 (intervention group). † n = 36 (control group), n = 36 (intervention group). ‡ n = 12 (control group), n = 18 (intervention group).
|
Box 3 –
Linear regression analysis of the relationship between modified Iowa Level of Assistance (mILOA) score and group allocation, after adjusting for relevant confounders (sex, anaesthetic type and home setting)
|
|
Unstandardised coefficient for mILOA score (standard error)
|
P
|
|
|
Group allocation (intervention v control)
|
–4.12 (1.99)
|
0.04
|
|
Sex (men v women)
|
–0.11 (1.96)
|
0.95
|
|
Anaesthetic type (general v spinal)
|
–6.59 (2.64)
|
0.02
|
|
Carer at home (v no carer at home)
|
1.25 (1.95)
|
0.52
|
|
Stairs at home (v no stairs at home)
|
–1.53 (2.13)
|
0.48
|
|
|
|
Box 4 –
Linear regression analysis of the relationship between hospital length of stay and group allocation, after adjusting for relevant confounders (sex, anaesthetic type and home setting)
|
|
Unstandardised coefficient for length of stay (standard error)
|
P
|
|
|
Group allocation (intervention v control)
|
–16.80 (4.90)
|
0.001
|
|
Sex (men v women)
|
7.64 (4.83)
|
0.12
|
|
Anaesthetic type (general v spinal)
|
–15.60 (6.52)
|
0.02
|
|
Carer at home (v no carer at home)
|
–10.06 (4.82)
|
0.04
|
|
Stairs at home (v no stairs at home)
|
4.01 (5.26)
|
0.45
|
|
|
|
Box 5 –
Adjusted Kaplan–Meier analysis of the probability of discharge, after adjusting for sex, anaesthetic type, carer at home, and stairs at home*
Box 6 –
Pain and pain management scores for the usual care (control) and intensive physiotherapy (intervention) groups during the first 3 post-operative days
|
Outcome
|
Usual care
|
Intensive physiotherapy
|
P
|
|
|
Number of patients
|
46
|
46
|
|
|
Post-physiotherapy pain scores, mean (SD) (maximum score: 10)
|
|
|
|
|
Day 1
|
5.3 (0.5)
|
4.3 (0.4)
|
0.16
|
|
Day 2
|
4.2 (0.5)
|
4.7 (0.5)
|
0.44
|
|
Day 3
|
4.0 (0.5)
|
4.2 (0.5)
|
0.79
|
|
Opioid equivalence score, mean (SD)*
|
|
|
|
|
0–24 hours post-operative
|
20.0 (14.0)
|
20.9 (15.8)
|
0.81
|
|
24–48 hours post-operative
|
26.3 (16.6)
|
34.1 (26.7)
|
0.12
|
|
48–72 hours post-operative
|
27.0 (19.8)
|
29.2 (22.9)
|
0.67
|
|
|
* Reference analgesic is oral morphine (mg).
|

 more_vert
more_vert