The known Angiography rates vary across Australia. Whether this variation is correlated with indices of socio-economic deprivation, chronic disease, acute coronary syndrome (ACS) incidence, or health service characteristics is uncertain.
The new Social disadvantage and remoteness were correlated with ACS incidence and mortality, but not with angiography rates. Private hospital cardiac admissions were strongly correlated with angiography rates; the relationship with public hospital cardiac admissions was less marked. Socio-economic indicators, regional location, and ACS and chronic disease burden were not significantly associated with angiography rates.
The implications A focus on clinical care standards and better health service distribution is needed to reduce the variation.
Managing coronary artery disease (CAD) with coronary angiography is informed by an extensive evidence base.1–5 Variation in the rates of coronary angiography has been described in Australia.6,7 This variation may be explained by heterogeneity in clinical need (ie, variations in disease incidence or prevalence), but differences unexplained by disease burden highlight inequities in access to health services, and differential over- and underuse of health care resources. These disparities are potentially targets for policy interventions that aim to improve population health and achieve a high quality health system.
Health care in Australia faces a combination of challenges. The geographic distribution of the population and substantial cultural diversity give rise to complexity in providing access to clinical expertise and procedures such as angiography. Clinical audits of acute coronary syndrome (ACS) practice have identified variations in applying components of care advocated in guidelines, particularly invasive management.8–12 Australia’s demography may contribute to heterogeneity in access to health services, differential clinical needs and variation in care. The relative contributions of demographic factors have implications both for national policy and for local efforts to re-design health services.
In our study we explored the associations of selected socio-economic, geographic, and chronic disease factors with ACS incidence and mortality rates, and examined whether rates of coronary angiography across the Australian population are correlated with indicators of disease burden, health access, and clinical activity.
Methods
Data sources
Socio-economic and health workforce data
Our analysis included data for the entire Australian population of about 23.5 million people. Between 2010 and 2015, federal government support for primary health care services was organised into 61 geographic divisions (Medicare Locals). Social, economic and health service characteristics of the Medicare Locals were obtained from a publicly accessible website that publishes age- and sex-standardised rates for these features, including the estimated proportions of privately insured residents and of Indigenous residents, based on data from the 2011 Australian census (conducted by the Australian Bureau of Statistics [ABS]).13 Data from the Socio-Economic Indexes for Areas (SEIFA),14 a relative measure of social and economic disadvantage (normalised to 1000; higher numbers denote more advantaged areas), were also used as a socio-economic indicator. Medical workforce data were drawn from the annual survey of the Australian Health Practitioner Registration Authority, which records the primary locations of practice and specialisations of all registered medical practitioners. Further, modelled rates of chronic cardiovascular conditions (prior myocardial infarction and angina, heart failure, stroke and rheumatic heart disease), estimates derived from the Australian Health Survey 2011–2013 (conducted by the ABS), were available for all but three Medicare Locals (Tasmania, Northern Territory, Australian Capital Territory).
As an indicator of local clinical practice in each region, the likelihood that a suspected ACS patient received coronary angiography, compared with the national average, was estimated from the SNAPSHOT ACS clinical audit.9,10
Rates of ACS, angiography, revascularisation and mortality
The National Hospital Morbidity Database (NHMD) was used to identify ACS separations (International Classification of Diseases, revision 10, Australian modification [ICD-10-AM] principal diagnosis codes I20 and I21) and catheter procedures during the calendar year 2011. Data on procedures undertaken as ambulatory care (eg, outpatient angiography) were obtained by the Australian Institute of Health and Welfare (AIHW) from the Medicare Benefits Schedule. The combined total rate of angiography is presented in this article. All cardiac-specific admissions were included in the analysis. Three of the eight state or territory jurisdictions did not report data for private hospital admissions, so that private hospital and total admissions were not available for three Medicare Locals (Tasmania, NT, ACT). Data on deaths attributed to CAD were drawn from the National Mortality Database (NMD).
Statistical analysis
Standardised age- and sex-specific rates — of ACS separations, inpatient and outpatient angiograms, percutaneous coronary interventions (PCI), coronary artery bypass graft (CABG) procedures, and CAD-related deaths of people aged 35 years or more grouped in 5-year age intervals (to 85 years or more) and by sex — were calculated by dividing by the corresponding Australian population figure (at 30 June 2001) for the relevant age and sex group, and expressed as numbers per 100 000 population.
Socio-economic data, health service information, procedure and ACS incidence, and mortality rates were stratified by geographic location of the Medicare Local as defined by the AIHW, and compared using Kruskal–Wallis tests. Univariate correlations between potential explanatory factors and rates of angiography, ACS and mortality were assessed using partial correlations; the strength of the adjusted correlation coefficient (CC) was defined as strong (> 0.70), moderate (0.50–0.69), or weak (0.30–0.49). Separate correlation plots of ACS v CAD mortality rates and of angiography v ACS rates were generated, and fitted linear predictions superimposed. To assess the potential overuse of angiography, the ratio of the numbers of coronary revascularisations to those of coronary angiograms was calculated for each Medicare Local, and plotted as a function of the rate of coronary angiography, using the fractional polynomial estimate.
Given the small numbers of Medicare Locals and the availability of earlier data to evaluate these relationships, a Bayesian linear regression approach was used (Appendix). All analyses were undertaken in Stata 14.0 (StataCorp); P < 0.05 was deemed statistically significant.
Ethics approval
This SNAPSHOT ACS study was conducted with local ethics approval for opt-out consent, except for two hospitals where opt-in consent was applied (lead ethics committee: Cancer Council NSW Human Research Ethics Committee; reference, 2011/06/334). The AIHW work program and any release of data from AIHW datasets are subject to the oversight of the AIHW Ethics Committee and handled in accordance with the AIHW Act 1987 (as amended), the Privacy Act 1988, and any terms and conditions set by data providers. Separate ethics committee approval is not required for the analysis of AIHW datasets that does not involve data linkage, and was thus not needed for our study.
Results
Characteristics of the Medicare Locals
Of the 61 Medicare Locals, 27 were categorised by AIHW criteria as metropolitan, 24 as regional and ten as rural. Regional and rural locations were significantly more disadvantaged than metropolitan areas, with higher proportions of the population on long term unemployment support, greater reported delays in seeking medical consultation because of the associated costs, and lower proportions of residents with private health insurance. Similarly, the prevalence of smoking, obesity and chronic cardiovascular disease was higher in regional and rural areas than in metropolitan areas. There was no difference in the rates of total hospital admissions by geographic location, but there was a significantly lower rate of private hospital cardiac admissions in non-metropolitan locations, where fewer private hospitals exist (Box 1).
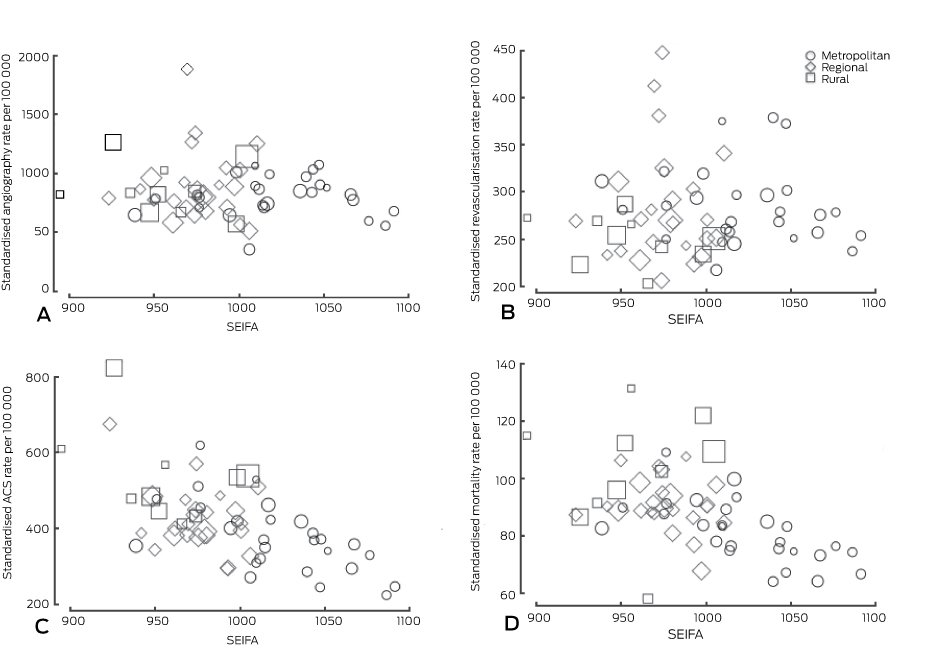
ACS rates were higher in non-metropolitan areas, but this was not reflected in a significantly higher rate of coronary angiography. PCI rates were significantly lower in rural areas, while rates of CABG were significantly higher in non-metropolitan areas. Overall, there was no significant difference in the combined coronary revascularisation rates according to geographic location. Premature death from CAD and total mortality were higher in regional and rural areas. There were 3.7-fold (ACS), 5.3-fold (angiography), 2.2-fold (revascularisation) and 2.3-fold (CAD mortality) differences between the lowest and highest rates for individual Medicare Locals. Box 2 shows the variation in ACS, angiography, revascularisation, and mortality rates for each Medicare Local by SEIFA index score.
Correlation of socio-economic, chronic health and health service data with ACS admission mortality rates
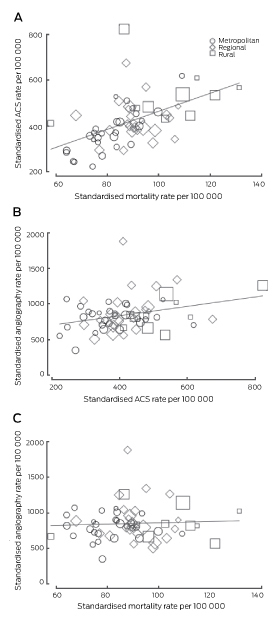
The ACS admission rates for individual Medicare Locals were significantly correlated with all-cause mortality (CC, 0.52; P < 0.001). There were strong correlations between socio-economic measures and ACS admission rates and mortality (Box 3). Similarly, rates of smoking, obesity and chronic cardiovascular conditions were all correlated with mortality; smoking and obesity rates were also correlated with ACS admission rates. Strong negative correlations between the proportion of insured people in the Medicare Local and the ACS admission and all-cause mortality rates were also observed. Conversely, there were negative correlations between local availability of specialist physicians and ACS admissions and mortality, as well as positive correlations between delays in consultations and these outcomes. From a health service use perspective, emergency department and public hospital admission rates were correlated with ACS admission and mortality rates, while private hospital cardiac admission rates were negatively associated with total mortality. An increased likelihood of patients admitted with suspected ACS undergoing angiography was associated with lower mortality (Box 3).
An adjusted analysis of mortality was performed to explore relationships with the indicators SEIFA score, regional location, local cardiovascular health status (chronic cardiovascular conditions), ACS rates, workforce capacity (access to specialist physician care), and health service provision (cardiac admission rates to public and private hospitals). Rural location was associated with increased mortality (19 additional deaths [95% CI, 10–27] per 100 000 population). Interestingly, the likelihood of coronary angiography in the context of ACS appeared to be associated with a modest reduction in mortality rates (three fewer deaths [95% CI, 1–5] per 100 000 population for each 10 percentage point increase in the likelihood of angiography for a suspected ACS admission). After considering these two factors, socio-economic index, disease burden, health service indicators, and angiography rates were not significantly correlated with the Medicare Local CAD mortality rate.
Correlation of socio-economic status, chronic health status and health service with angiography rates
There was no correlation between measures of social disadvantage or health service availability and coronary angiography rates (Box 3). A positive correlation between all cardiac admissions and angiography rates was evident, particularly private hospital cardiac admissions. This correlation was weaker when analysis was restricted to public hospital admissions. The likelihood of angiography for acute patients was not correlated with the overall rate of angiography in Medicare Locals. There was a weak correlation between the rates of angiography and of ACS (CC, 0.31; P = 0.018), but no correlation with premature ischaemic heart disease deaths (CC, 0.13; P = 0.315) or total CAD mortality (CC, 0.06; P = 0.671) (Box 4).
In the adjusted model, private hospital cardiac admissions had a large influence on the angiography rate (71 additional angiograms [95% CI, 47–93] per 1000 admissions). The relationship between public hospital admission rates and the angiography rate was more modest (44 angiograms [95% CI, 25–63] per 1000 admissions). Socio-economic indicators, regional location, and background ACS or chronic disease rate burden were not significantly associated with angiography rates.
Progression from angiography to revascularisation
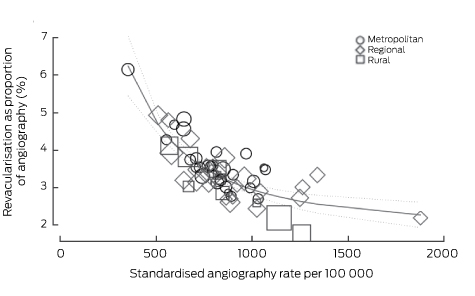
The angiography rate was correlated with those of PCI (CC, 0.54; P < 0.001), CABG surgery (CC, 0.44; P < 0.001) and any revascularisation (CC, 0.65; P < 0.001). Revascularisation rates as a proportion of angiography rates varied greatly (17–61%). There was a striking negative correlation between the angiography rate and the proportion of patients undergoing angiography who proceeded to any form of coronary revascularisation (CC, −0.71; P < 0.001) (Box 5). This was also seen with the individual modes of revascularisation (PCI: CC, −0.62; P < 0.001; CABG: CC, –0.62; P < 0.001). There was no correlation between PCI rates and the incidence of myocardial infarction (CC, −0.11; P = 0.402) or ACS (CC, −0.12; P = 0.345). However, rates of CABG were correlated with those of myocardial infarction (CC, 0.53; P < 0.001) and of ACS (CC, 0.45; P < 0.001).
Discussion
We observed that:
-
increasing socio-economic disadvantage, rural location, and chronic disease burden were each correlated with rates of ACS and of total mortality;
-
local availability of specialist physicians and admissions to private hospitals were negatively correlated with ACS admissions and mortality rates;
-
there was no association between coronary angiography rates and the burden of chronic cardiovascular disease, and a modest positive association with ACS rates, but there was a positive association between angiography rates and those of cardiac admissions to private hospitals; and
-
the rates of angiography and coronary revascularisation were correlated, but there was a negative correlation between the local angiography rates and the proportion of these procedures proceeding to revascularisation.
These findings suggest that health reforms aimed at the appropriate use of diagnostic coronary angiography may be required to improve consistency and equity of access, and consequently to deliver positive outcomes for the Australian community more efficiently.
As expected, a correlation between the incidence of ACS and CAD mortality was evident. Similarly, higher ACS rates in regional and rural locations were confirmed, as was the relationship between indicators of socio-economic disadvantage and chronic cardiac disease burden, and between disease incidence and outcomes. However, the distribution of acute care services was negatively correlated with the incidence of disease in the population. This geographic mismatch has also been described elsewhere, including the United States, and highlights the influence of factors such as funding and workforce proficiency in delivering services to non-metropolitan communities.15–17
A solid evidence base supports using coronary angiography, with subsequent revascularisation where deemed appropriate, in ACS patients with elevated troponin levels.18 However, the superiority of angiography and revascularisation to medical management of patients with stable CAD is less robust.5,19 This clinical evidence base contrasts strongly with several of our findings. Firstly, the indicator most associated with variation in angiography appeared to be cardiac admission rates to private hospitals, but an inverse correlation with ACS rates implies that these procedures are mainly being undertaken for indications other than ACS. Secondly, while angiography rates were correlated with revascularisation rates, neither angiography nor PCI rates (unlike the CABG rate) were correlated with that of ACS. Thirdly, angiography rates were negatively correlated with progression to revascularisation. These observations are inconsistent with the evidence base for invasive management for non-ACS indications, with private institutions accounting for a higher proportion of the variation. Computed tomography coronary angiography for investigating non-acute CAD may reduce the use of invasive angiography for this indication, but caution should be exercised, as there is ample evidence that all forms of cardiac testing tend to motivate further investigations.19,20
These comparisons suggest certain policy targets for improving the clinically appropriate application of coronary angiography. At the higher end of patient risk, the Australian Commission on Safety and Quality in Health Care has developed clinical care standards for the management of ACS.21 These standards may appropriately increase the use of angiography for ACS patients, for whom the benefits of invasive management have been established. Linking the funding of hospitals to ACS performance measures may be an approach for achieving changes in practice and outcomes. Further, resourcing and implementing clinical support networks that serve regional and remote areas (eg, telemedicine) would enable the appropriate selection of patients who would benefit most from being transferred for angiography and revascularisation, and this would also be an opportunity for improving access to angiography. While there is no current guidance on stable CAD in Australian, criteria for the appropriateness of angiography and revascularisation have been developed in the US.22 It is notable that re-imbursement by Medicare and Medicaid in the US for the costs of invasive procedures is now linked to these appropriateness criteria. It is suggested that funding linked to the appropriateness of care or the achievement of clinical care standards, and limiting re-imbursement for angiography in low value clinical situations, should be focuses of debate in any health care reform discussion in Australia.
Limitations
Given the ecological study design, pockets of excellence in care and outcomes probably exist but are obscured by data aggregation. Further, in view of the small number of Medicare Locals, a linear relationship between variables was assumed, although curvilinear relationships are possible. The small sample of the SNAPSHOT ACS study is acknowledged, with caution accordingly exercised when interpreting the association with mortality rates. While this relationship should be examined in larger studies, our finding is consistent with international large scale data.23 Similarly, we cannot fully exclude the possibility of under- (misclassification) or over-reporting (double counting secondary to inter-hospital transfers) of ACS admissions or procedures; systematic under-reporting is, however, unlikely, given the funding implications of these coding practices. Detailed interrogation of the system would require documentation of patient-level characteristics and care, combined with an evaluation of the health service infrastructure beyond the availability of catheter laboratories, extending to other modalities of cardiac investigation, such as computed tomography coronary angiography and functional imaging. Such information is not currently available in Australia.
Conclusion
Significant variation in providing coronary angiography, not related to clinical need, is evident across Australia. A greater focus on clinical care standards and better distribution of health services will be required if these disparities are to be reduced.
Box 1 –
Socio-economic and health service characteristics, and age- and sex-standardised rates of death, diagnosis, and coronary procedures (per 100 000 population), by Medicare Locals stratified according to metropolitan, regional and rural locations, for the calendar year 2011
|
|
Total
|
Metropolitan
|
Regional
|
Rural
|
P
|
|
|
Number
|
61
|
27
|
24
|
10
|
|
|
Socio-economic indicators
|
|
|
|
|
|
|
SEIFA score, mean (SD)
|
992 (42)
|
1022 (39)
|
976 (21)
|
955 (33)
|
0.001
|
|
Indigenous population, mean (SD)
|
3.9% (5.5)
|
1.1% (0.7)
|
3.1% (2.1)
|
13.2% (8.1)
|
0.001
|
|
Long term unemployed, mean (SD)
|
3.5% (1.4)
|
2.6% (1.1)
|
4.1% (1.0)
|
4.6% (1.5)
|
0.001
|
|
Private insurance, mean (SD)
|
44.3% (9.9)
|
51.7% (9.3)
|
40.8% (4.3)
|
33.0% (5.2)
|
0.001
|
|
Chronic health status indicators
|
|
|
|
|
|
|
Diabetes, mean (SD)
|
5.3% (1.0)
|
5.7% (1.1)
|
4.8% (0.7)
|
5.5% (0.9)
|
0.004
|
|
Hypertension, mean (SD)
|
10.2% (0.6)
|
10.2% (0.6)
|
10.3% (0.6)
|
10.1% (0.5)
|
0.994
|
|
Smokers, mean (SD)*
|
19.1% (3.6)
|
16.2% (2.9)
|
21.4% (1.8)
|
22.8% (1.5)
|
0.001
|
|
Obesity, mean (SD)*
|
28.3% (4.2)
|
25.5% (4.4)
|
30.6% (1.9)
|
31.4% (2.1)
|
0.001
|
|
Hypercholesterolaemia, mean (SD)
|
33.1% (1.7)
|
32.9% (1.4)
|
33.6% (1.8)
|
32.1% (2.2)
|
0.186
|
|
Chronic cardiovascular condition, mean rate (SD)*
|
88 (14)
|
81 (11)
|
91 (9)
|
102 (21)
|
< 0.001
|
|
Premature ischaemic heart disease, mean rate (SD)
|
28.4 (10.2)
|
22.9 (5.1)
|
27.6 (3.4)
|
45.3 (13.3)
|
< 0.001
|
|
Access and health workforce indicators
|
|
|
|
|
|
|
Delay in medical consultation because of cost, mean (SD)
|
14.6% (3.6)
|
13.2% (3.8)
|
15.3% (2.9)
|
16.8% (3.1)
|
0.006
|
|
Primary care physicians, mean rate (SD)
|
110.8 (17.4)
|
113.9 (22.1)
|
109.1 (10.8)
|
106.3 (15.8)
|
0.776
|
|
Specialist physicians, mean rate (SD)*
|
22.9 (21.6)
|
34.2 (26.7)
|
13.4 (6.5)
|
10.8 (6.4)
|
0.002
|
|
Health service provision indicators
|
|
|
|
|
|
|
Primary care health check, mean rate (SD)
|
4266 (1181)
|
4336 (1034)
|
4548 (1239)
|
3401 (1111)
|
0.032
|
|
Public cardiac admissions, mean rate (SD)
|
1684 (451)
|
1366 (274)
|
1826 (316)
|
2201 (479)
|
< 0.001
|
|
Private cardiac admissions, mean rate (SD)†
|
752 (264)
|
861 (202)
|
717 (283)
|
527 (227)
|
0.021
|
|
All emergency department presentations, mean rate (SD)
|
30 881 (11 358)
|
24 939 (11 358)
|
32 400 (9837)
|
43 277 (17 082)
|
< 0.001
|
|
Likelihood of angiogram in suspected acute coronary syndrome, mean (SD)
|
40.6% (16.7)
|
49.2% (17.2)
|
30.9% (8.9)
|
41.4% (18.2)
|
< 0.001
|
|
Coronary events and procedures
|
|
|
|
|
|
|
Myocardial infarction, mean rate (SD)
|
250 (63)
|
225 (48)
|
250 (46)
|
316 (90)
|
0.003
|
|
Acute coronary syndrome, mean rate (SD)
|
419 (108)
|
375 (93)
|
423 (84)
|
532 (120)
|
< 0.001
|
|
Coronary angiography, mean rate (SD)
|
849 (236)
|
803 (167)
|
895 (300)
|
863 (218)
|
0.742
|
|
Percutaneous coronary intervention, mean rate (SD)
|
212 (47)
|
222 (38)
|
215 (58)
|
178 (23)
|
0.009
|
|
Coronary artery bypass surgery, mean rate (SD)
|
70 (15)
|
64 (13)
|
74 (15)
|
75 (18)
|
0.040
|
|
Revascularisation, mean rate (SD)
|
278 (49)
|
284 (41)
|
283 (61)
|
250 (25)
|
0.089
|
|
Premature ischaemic heart disease deaths, mean rate (SD)‡
|
28.4 (10.2)
|
22.9 (5.1)
|
27.6 (3.4)
|
45.3 (13.3)
|
< 0.001
|
|
Premature cerebrovascular accident deaths, mean rate (SD)‡
|
9.2 (2.4)
|
8.2 (1.6)
|
9.4 (1.7)
|
11.5 (3.7)
|
< 0.001
|
|
Total mortality, mean rate (SD)
|
88 (14)
|
81 (11)
|
91 (9)
|
102 (21)
|
< 0.001
|
|
Population, mean (SD)
|
296 666 (165 595)
|
414 767 (144 115)
|
232 023 (110 353)
|
132 939 (94 442)
|
< 0.001
|
|
|
SEIFA = Socio-Economic Indexes for Areas. * Estimates from modelled data: not available for three Medicare Locals (all rural). † Data not released for three Locals (one each for metropolitan, regional and rural). ‡ Annualised rates per 100 000 individuals for 2008–2011.
|
Box 2 –
Variation in angiography, revascularisation, acute coronary syndrome and mortality rates according to Socio-Economic Index for Australia score for the location of the Medicare Local*
Box 3 –
Correlations between indicators of socio-economic status, health status, health workforce, health care access and clinical practice, and coronary angiography, acute coronary syndrome and mortality rates in Medicare Locals*
|
|
Coronary angiography rate
|
Acute coronary syndrome admission rate
|
Total mortality rate
|
|
|
Socio-economic indicators
|
|
SEIFA score
|
–0.11 (0.42)
|
–0.62 (< 0.001)
|
–0.54 (< 0.001)
|
|
Indigenous population†
|
–0.08 (0.53)
|
0.53 (0.002)
|
0.30 (0.019)
|
|
Long term unemployed†
|
–0.07 (0.62)
|
0.60 (< 0.001)
|
0.46 (0.002)
|
|
Private insurance†
|
–0.15 (0.24)
|
–0.65 (< 0.001)
|
–0.62 (< 0.001)
|
|
Chronic health status indicators
|
|
Diabetes†
|
–0.22 (0.10)
|
–0.05 (0.72)
|
0.001 (0.94)
|
|
Hypertension†
|
–0.27 (0.03)
|
–0.17 (0.20)
|
0.16 (0.22)
|
|
Smokers†
|
0.25 (0.05)
|
0.61 (< 0.001)
|
0.62 (< 0.001)
|
|
Obesity†
|
0.15 (0.26)
|
0.51 < 0.001)
|
0.65 (< 0.001)
|
|
Hypercholesterolaemia†
|
0.16 (0.23)
|
–0.39 (0.002)
|
–0.08 (0.54)
|
|
Chronic cardiovascular condition†
|
–0.21 (0.12)
|
0.05 (0.70)
|
0.38 (0.003)
|
|
Premature ischaemic heart disease
|
0.13 (0.32)
|
0.59 (< 0.001)
|
0.58 (< 0.001)
|
|
Access and health workforce indicators
|
|
Delay in medical consultation because of cost†
|
0.05 (0.69)
|
0.61 (< 0.001)
|
0.45 (< 0.001)
|
|
Primary care physicians
|
–0.07 (0.59)
|
–0.26 (0.04)
|
–0.39 (0.002)
|
|
Specialist physicians
|
0.12 (0.37)
|
–0.41 (0.002)
|
–0.47 (< 0.001)
|
|
Health service provision indicators
|
|
Primary care health check (45 years)
|
0.28 (0.03)
|
0.02 (0.88)
|
–0.12 (0.35)
|
|
Public cardiac admissions
|
0.30 (0.02)
|
0.65 (< 0.001)
|
0.49 (< 0.001)
|
|
Private cardiac admissions
|
0.44 (0.006)
|
–0.13 (0.32)
|
–0.42 (< 0.001)
|
|
Emergency presentations
|
0.14 (0.28)
|
0.47 (0.001)
|
0.35 (0.005)
|
|
Likelihood of angiogram in suspected acute coronary syndrome†
|
0.06 (0.69)
|
–0.01 (0.96)
|
–0.40 (0.002)
|
|
|
SEIFA = Socio-Economic Indexes for Areas. * Expressed as correlation coefficient, with significance (P) in parentheses. † Correlation between percentage of population positive for indicator and angiography, acute coronary syndrome admissions and mortality rates.
|
Box 4 –
Correlation between acute coronary syndrome and mortality rates, angiography and acute coronary syndrome admissions rates, and angiography and mortality rates*
Box 5 –
Revascularisation rates as proportions of angiography rates in each Medicare Local,* with fractional polynomial estimate and 95% confidence band

 more_vert
more_vert