This report presents data on the Indigenous children and young people who participated in the audiology, ear, nose and throat (ENT) teleotology and Clinical Nurse Specialist (CNS) services delivered under the National Partnership Agreement on Northern Territory Remote Aboriginal Investment. During 2012–16, 9,221 outreach audiology services were provided to 5,357 children and young people, and 3,799 ENT teleotology services to 2,434 children and young people. A total of 2,612 children participated in the CNS services and presented for 3,085 visits. Of the children and young people who received audiology services in 2015–16, 31% had a hearing impairment.
Preference: Administration and Health Services
2
Flu vaccine not available until April 2017
The Federal Government has announced that the quadrivalent influenza vaccine stocks won’t hit GPs until mid-April this year.
According to a Health department spokeswoman, “The vaccine companies are finding it harder each year, with some many strain changes, to produce enough vaccine for mass distribution before April.“
Although flu vaccines are being administered in some chemists from mid March, there is an advantage to a slightly later vaccination date.
According to Australian Technical Advisory Group on Immunisation (ATAGI) advice, “Recent evidence suggests protection against influenza may start to decrease from 3 to 4 months following vaccination and early vaccination needs to be balanced with this.”
The peak month for influenza in Australia is August and it’s estimated to be responsible for more than 5000 hospitalisations and almost 170 deaths each year.
The strains covered in this year’s vaccination are:
- A (H1N1): an A/Michigan/45/2015 (H1N1)pdm09* like virus
- A (H3N2): an A/Hong Kong/4801/2014 (H3N2) like virus
- B: a B/Brisbane/60/2008 like virus
- B: a B/Phuket/3073/2013 like virus
Typically, people most commonly affected are young children and older adults with pregnant women particularly high risk of becoming seriously ill.
The immunisation vaccine is funded under the National Immunisation Program for certain at risk groups. They are:
- Aboriginal and/or Torres Strait Islander children between 6 months and 5 years and 15 years and over,
- Anyone 65 years and over,
- Anyone 6 months and over who have certain medical conditions including severe asthma, lung or heart disease, low immunity or diabetes,
- Pregnant women.
For more information, visit the: Australian Technical Advisory Group on Immunisation (ATAGI) advice for immunisation providers regarding the administration of seasonal influenza vaccines in 2017 and the updated Australian Immunisation Handbook 10th edition.
Latest news
Variation in outpatient consultant physician fees in Australia by specialty and state and territory
The known Concerns have been expressed about high consultation fees and the potential for a two-tiered health system developing in Australia. Insurers are prohibited from covering outpatient physician consultation charges.
The new There were wide variations in bulk-billing rates and fees within specialties, between specialties, and between states and territories. Out-of-pocket payments by patients varied more than fivefold in some specialties.
The implications There is a lack of transparency and public availability of information about charges for outpatient consultations. Without data on quality of care, the justifiability of the differences in fees cannot be determined.
About 2.4 million initial consultations with consultant physicians are conducted in Australia each year.1 The Medicare program provides a set payment (rebate) for specific clinically relevant services to offset the cost to patients of private consultations.2 This payment is based on the “schedule fee”, the amount determined by the Department of Health as being reasonable, on average, for the service provided.2 The schedule fee for each eligible service is published in the Medicare Benefits Schedule (MBS). Each year, Medicare provides about $300 million in rebates for initial appointments with consultant physicians, accounting for about 1.5% of total Medicare benefits.3
The schedule fee is stated to reflect the difficulty and time involved in providing the service, as well as factors such as major capital costs, and direct (eg, consumables) and indirect costs (eg, salaries for administrative staff).4 Medicare provides a rebate to patients of 85% of the schedule fee for most outpatient appointments.2 Doctors may choose to accept the 85% benefit amount as the full payment; this is known as bulk-billing and results in there being no cost to the patient.2 About 41% of medical and surgical outpatient specialist or consultant attendances were bulk-billed during the September quarter of 2016.5 “Specialist” and “consultant physician” are Medicare terms used in item descriptions, and refer to medical practitioners recognised as either specialists or consultant physicians for the purposes of the Health Insurance Act 1973.
Doctors have expressed concern that increases in fee levels in the MBS have not kept pace with inflation and do not reflect appropriate payments for services.6–8 Specialists and consultants in private practice are permitted to charge more than the schedule fee,9 and can charge any rate they feel the market will bear.10 MBS data show that 56.1% of outpatient specialist and consultant physician appointments are charged at rates higher than the schedule fee.5
Since 1983, legislation has prohibited private health insurers from covering outpatient visits.11,12 The patient is therefore responsible for paying any difference between the fee charged by the doctor and the Medicare rebate for any non-bulk-billed service. This difference, the out-of-pocket expense or gap fee, is usually paid by the patient at the time of service delivery.10 There is a large degree of variation in fees charged by specialists and consultant physicians across geographic areas,5 as well as between specialties.
In 2009–10, Australian households spent an average of $325 annually on out-of-pocket expenses for specialist and consultant physician consultations.13 The amount varied considerably between states; it was lowest in Tasmania ($125 per household) and highest in the Australian Capital Territory ($554 per household).13 A study during 2013–14 found that 7.9% of people who needed to see a specialist or consultant physician delayed the appointment or did not go at all because of the expense.14 This economic barrier to access is more important for people living in areas of socio-economic disadvantage or outside capital cities, as well as for those with long term health problems.15
Concerns about the high levels of medical appointment fees and the limited ability of many Australians to pay for care have recently been raised in the media and by professional groups (including the Royal Australasian College of Surgeons).15 A report by a private health insurance company identified marked variation in fees for inpatient procedures, with some surgeons charging almost $17 000 more than other surgeons for a particular operation.16 As private health insurers are prohibited from reimbursing outpatient consultation fees, there are no data for such services according to specialty, reducing the transparency of charging practices.
Publicly available data indicate that the average out-of-pocket charge for private outpatient medical specialist or consultant physician appointments is $71.90.5 However, fee differences between medical specialties and between states/territories have not been examined.
As waiting times for public specialty care have increased in Australia, the number of patients seeking care in the private sector has also increased. Greater transparency in consultation charges, particularly regarding differences between states and specialties, would benefit patients and provide information about the actual costs to the patient of private outpatient care. Our study examined the actual fees charged for an initial outpatient consultation (Medicare item number 110) by consultant physicians in different specialties, and calculated the out-of-pocket costs for these services. The schedule fee (January 2016) for an initial appointment with a consultant physician was $150.90 and the benefit (rebate) amount was $128.30.2
Methods
We analysed aggregated, non-identifiable Medicare data from the Commonwealth Department of Human Services (DHS) on Medicare claims for item number 110 (an initial appointment with a consultant physician following a referral2) rendered between 1 January 2015 and 31 December 2015. The data file was reviewed by the Department of Health before it was released to us. To prevent specific providers being identified, the DHS suppressed data when fewer than 20 services were provided in a specific specialty in a state or territory.
The data provided by the DHS included the number of initial outpatient medical consultations (Medicare item number 110) for which a claim for benefit was made. These included separate data for the absolute number of bulk-billed and non-bulk-billed visits. Whenever a Medicare claim is made for a non-bulk-billed service, the actual charge for the visit must be provided to the DHS as part of the claim. The DHS data included the mean, median, and 10th and 90th percentile levels of actual charges by doctors. These data were aggregated by medical specialty and state or territory of the doctor providing the service.
We analysed data from 11 medical specialties representative of non-surgical medical care provided to adults by consultant physicians: cardiology, endocrinology, gastroenterology, geriatric medicine, haematology, immunology/allergy, medical oncology, nephrology, neurology, respiratory medicine, and rheumatology. We report only consultation charges for non-bulk-billed visits.
Ethics approval
This study received ethics approval from the University of Melbourne Human Research Ethics Committee (reference, 1646466.1).
Results
Proportion of consultations bulk-billed, by specialty and state
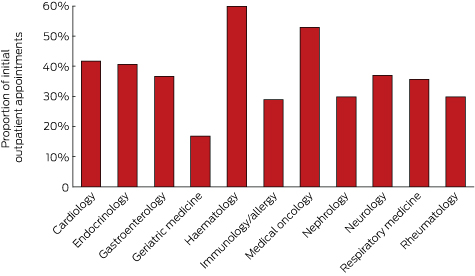
The specialties with the highest proportion of bulk-billed initial consultations (Medicare item 110) were haematology and medical oncology. The lowest bulk-billing rates were in geriatric medicine. Most specialties bulk-billed 30–42% of visits (Box 1).
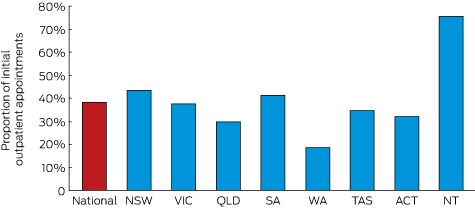
The state or territory with the highest overall bulk-billing rate was the Northern Territory (76% of visits); New South Wales and South Australia also had bulk-billing rates above 40%. Only Western Australia had a bulk-billing rate under 20% (Box 2).
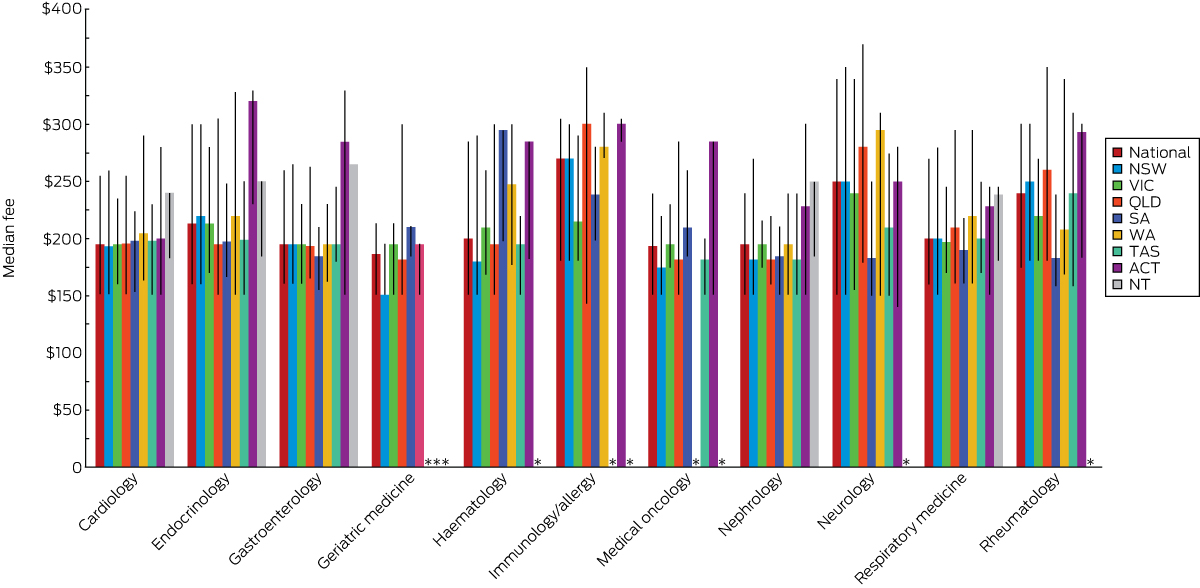
Fees for initial outpatient consultation: by specialty
There was marked variation within and between specialties, as well as between states and territories, in the mean, median and 10th/90th percentile levels of fees for an initial outpatient consultation with a consultant physician.
Immunology/allergy was the specialty with the highest mean ($257) and median ($270) fees for an initial consultation, followed by neurology (mean, $252; median, $250). Mean fees for an initial consultation were less than $200 in only three of the 11 specialties (medical oncology, nephrology and geriatric medicine), with the lowest mean in geriatric medicine (Box 3).
There were four specialties for which the 90th percentile fee level was at least $300 for an initial consultation: neurology ($340), immunology/allergy ($305), rheumatology ($300), and endocrinology ($300). The 10th percentile fee level for nine specialties was $160 or less; the exceptions were immunology/allergy ($180) and rheumatology ($175).
The highest median out-of-pocket cost was for an initial consultation for immunology/allergy ($141.70), and the lowest was for geriatric medicine ($58.30; Box 3).
Variation in fees and out-of-pocket costs within specialties
There were also marked differences within specialties in the range of fees for an initial consultation. On average, the 90th percentile fee was 73% higher than that at the 10th percentile. The specialty with the greatest difference between the 10th and 90th percentile fee levels was neurology, where the difference between the upper and lower decile fees ($189) was equivalent to a 125% difference in fee; the narrowest range was the $62.50 difference (15%) for geriatric medicine. The 90th percentile out-of-pocket cost was more than 400% higher than the 10th percentile level for many specialties (Box 3).
Fees for initial outpatient consultation: by state and territory
Box 4 shows the variation in median fee for each specialty by state. The highest median fees for five of the 11 specialties were charged in the ACT, for three in the NT. Neither Victoria nor NSW had the highest median fees for any of the specialties.
Discussion
Our most important finding is the wide range of fees charged within specialties for an initial physician outpatient consultation. There is also variation within and between states. As there is no publicly available information about the quality of care in the outpatient setting or any validated outpatient quality measures, these fee variations are not based on any objective information about the care provided by individual doctors. Further, because information on the range of fees for outpatient consultations was not previously available, patients have not been aware that their out-of-pocket payments could vary markedly according to the private consultant physician they visit.
Because of increasing waiting times in the public sector,17,18 many patients choose private care so that they can see a specialist sooner. However, our data indicate that only a minority of these visits are bulk-billed, with the notable exceptions of consultations in haematology and medical oncology. Most patients must therefore make out-of-pocket payments to receive care in the private system. In contrast to inpatient care and many procedures, private health insurance coverage is not available for outpatient consultations, as private health insurance funds have been prohibited by law since 1983 from covering any difference between the schedule fee and the practitioner’s actual fee for outpatient visits.11 The rationale for this prohibition was the belief that allowing private health insurers to subsidise out-of-pocket costs might encourage practitioners to raise their fees further above the schedule fee, reducing access to specialist care for people who could not afford to either purchase private health insurance or to directly pay the higher fees.12 A reform was proposed to Parliament in 2003 that would have allowed private health insurers to cover outpatient costs that exceeded a $1000 annual threshold,11 but was not adopted.
The reasons for the variation in bulk-billing rates between states are not clear, with the exception of the relatively lower economic status of the population of the NT. There may be other economic factors affecting billing patterns in different states. Studies in other countries have found variations in the cultural norms of charging patterns of physicians in different communities.19 This phenomenon deserves further investigation.
Although the Medicare fee schedule was designed to be adjusted for inflation, there have been years in which no adjustment was made.7 This was usually the result of efforts to achieve federal budget savings, as increases in schedule fees would result in higher Medicare rebates and therefore higher government health care expenditure. However, one potential consequence of failing to increase schedule fees has been that doctors raise their own fees to keep pace with inflation and to increase their income.7 Over time, the gap between charged fees and the level of the rebate has grown, resulting in rising out-of-pocket charges.
For patients, limited funding of the public sector has resulted in longer waiting times for public outpatient consultations, leading to increased pressure to seek private care.18 Patients with limited means are faced with a difficult choice between delays for care in the public sector and out-of-pocket expenses in the private sector. There is consequently a risk that a two-tiered system of health care for outpatient consultations will develop.
It is unclear why the median and 90th percentile fees for immunology/allergy and neurology consultations are higher than for other initial outpatient consultant physician consultations. The duration of training for these specialties is no longer than for the other specialties we examined. It is important to note that the fee data in our report are for the same Medicare item number; the scheduled fee for item 110 is fixed, regardless of the duration of the consultation, and does not include any payment for a procedure, which must be billed separately when claiming a rebate. Some physicians may provide longer consultations than others, and this may explain some of the variance in fees within and between different specialties. As Medicare does not collect information on the duration of visits, data are not available for assessing this hypothesis.
There is also no clear rationale for the variation in median fees between states and territories. High fees for many consultant physician specialties in the ACT may reflect the economic status of the region or that of the patient population. Our findings for the NT might appear paradoxical: the bulk-billing rate in the NT was the highest in Australia, but some of the highest median fees were also charged here. It is possible that doctors in the NT bulk-bill the large number of patients with limited incomes, but, to compensate for the perceived reduction in income, charge those with the ability to pay much higher fees. Around the country, it is possible that some of the variation in fees is the result of physicians charging different patients different fees,20 but additional data would be required to determine the magnitude of this practice.
The lack of publicly available data about the range of fees for specific services places patients at a distinct disadvantage when seeking affordable medical care. Further, as there are no data on quality of care in the outpatient setting, patients are not only unsure about the range of appropriate fees, but also about the value of the service they receive. There are currently no requirements for private consultants to participate in quality assessments of their outpatient care, so patients cannot assess the association, if any, between fee level and quality of care. Other nations have developed programs for assessing the quality of outpatient care and make this information available to patients.21 Efforts of this type are needed in Australia as the health care system matures. Greater transparency in the fees charged by consultant physicians may have an impact that would benefit patients.
In light of our data, and the failure of increases in the MBS fee schedule to keep pace with inflation, the policy of prohibiting insurance coverage for outpatient care may need to be reconsidered. It appears that the goal of this policy — to limit outpatient fees — has met with limited success at best. The accessibility of private outpatient care for Australians is currently compromised by the level of out-of-pocket expense. Although private insurance has enabled many Australians to use private inpatient care, this has not been the case for outpatient consultations. It is possible that expanding insurance coverage to outpatient care would lead to a further increase in consultant fees. The primary goal of any changes in policy should be to improve access to consultants, whether in the public or private sectors. Additional research is essential for better understanding the variation in fees charged by consultant physicians and for informing future policies.
Box 1 –
Proportion of initial outpatient appointments with a consultant physician (Medicare item 110) in 2015 that were bulk-billed, by specialty
Box 2 –
Proportion of initial outpatient appointments with a consultant physician (Medicare item 110) in 2015 that were bulk-billed, by state and territory
Box 3 –
Consultation fees and patient’s out-of-pocket costs for initial non-bulk-billed outpatient appointment with a consultant physician, 2015
|
Specialty |
Fee |
Out-of-pocket costs |
|||||||||||||
|
Mean |
Median |
10th percentile |
90th percentile |
Mean |
Median |
10th percentile |
90th percentile |
||||||||
|
|
|||||||||||||||
|
Cardiology |
$202.00 |
$195.20 |
$150.90 |
$255.00 |
$73.70 |
$66.90 |
$22.60 |
$126.70 |
|||||||
|
Endocrinology |
$223.00 |
$213.40 |
$160.00 |
$300.00 |
$94.70 |
$85.10 |
$31.70 |
$171.70 |
|||||||
|
Gastroenterology |
$204.00 |
$195.20 |
$160.00 |
$260.00 |
$75.70 |
$66.90 |
$31.70 |
$131.70 |
|||||||
|
Geriatric medicine |
$185.00 |
$186.60 |
$150.90 |
$213.40 |
$56.70 |
$58.30 |
$22.60 |
$85.10 |
|||||||
|
Haematology |
$214.00 |
$200.00 |
$150.90 |
$285.00 |
$85.70 |
$71.70 |
$22.60 |
$156.70 |
|||||||
|
Immunology/allergy |
$257.00 |
$270.00 |
$180.00 |
$305.00 |
$128.70 |
$141.70 |
$51.70 |
$176.70 |
|||||||
|
Medical oncology |
$196.00 |
$193.85 |
$150.90 |
$240.00 |
$67.70 |
$65.55 |
$22.60 |
$111.70 |
|||||||
|
Nephrology |
$196.00 |
$195.00 |
$150.90 |
$240.00 |
$67.70 |
$66.70 |
$22.60 |
$111.70 |
|||||||
|
Neurology |
$252.00 |
$250.00 |
$151.00 |
$340.00 |
$123.70 |
$121.70 |
$22.70 |
$211.70 |
|||||||
|
Respiratory medicine |
$211.00 |
$200.00 |
$160.00 |
$270.00 |
$82.70 |
$71.70 |
$31.70 |
$141.70 |
|||||||
|
Rheumatology |
$236.00 |
$240.00 |
$175.00 |
$300.00 |
$107.70 |
$111.70 |
$46.70 |
$171.70 |
|||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Variation in the fees of medical specialists: problems, causes, solutions
Greater transparency in setting charges may be the most efficient way to rein in excessive fees
Articles in this issue of the MJA1,2 and elsewhere3–5 have reported significant variation in the fees charged by specialist physicians and surgeons. These variations raise questions about excessive health care costs, as well as about barriers to access for patients.2,4,5
That costs are barriers to medical care in Australia has been reported by 8% of people who needed to see a medical specialist and by 19% of those needing to see a general practitioner.5 International studies have also found that cost is a barrier for those needing medical specialist or general practice care; Australian prices generally fall in the middle range of the countries surveyed, but are among the highest for patients with certain chronic conditions.6 Financial barriers are more significant for poorer, sicker people, and for those in remote areas,7 and in the long run may lead to poorer health and greater costs.
The causes of fee variations are essentially market-related. Supply and demand conditions vary across geographic and specialty markets, and areas with fewer doctors are likely to face higher fees.7 Price variations are also a symptom of the uncompetitive nature of markets for certain medical specialist services, so that some doctors are able to charge well above the levels commonly charged for these services. Whether supply is adequate depends on demand, and this in turn depends on the patients’ clinical needs, their ability and willingness to pay (both strongly linked to income),3 and their ability to find alternative care (in public hospitals, for instance). Supply — the services that will be provided at a given price — depends on doctor numbers, the hours they are prepared to work, and on their attitudes to charging, including views on altruism.
The future supply of medical specialists will be influenced by the growing number of graduates from medical schools, subject to specialist training capacities.8 Increasing medical specialist numbers would be expected to lead to increased bulk-billing and lower average fees, but may also increase the volume of services. Other consequences may be difficult to predict, including changes to employment arrangements, such as greater willingness to become salaried medical officers.
Some of the proposed solutions for reducing excessive fees and high co-payments are unlikely to have a substantial impact. For example, there are calls to remove the Medical Benefits Schedule (MBS) fee freeze.2 However, evidence from general practice9 and the MBS safety net10 suggest that increasing the levels of rebates would have minimal impact on out-of-pocket costs, as doctors are aware of what price “the market will bear”.1 More importantly, such changes are unlikely to reduce variations within medical specialties, or to reduce the extreme out-of-pocket fees charged by some practitioners.
Other self-regulatory solutions, such as professional colleges offering education about “reasonable” fee-setting (eg, the Royal Australasian College of Surgeons Code of Conduct11) have, in practice, not constrained fees. Colleges may find this role easier were government price setting more transparent and rules for setting MBS schedule fees more explicit.
Easier access to information may induce greater competition. Patients have little opportunity to verify claims of higher quality care by medical specialists, and it is difficult to shop around to find the best price. Unverified quality claims can lead to extensive price variation, despite there being little evidence that quality is correlated with price.2 Information for patients could be improved by a website where medical specialists report their fees (eg, myDr.com.au, healthdirect.gov.au), enabling patients and referring GPs to make clinically and financially informed choices. Equally importantly, transparent pricing could lead to price competition between medical specialists.
Extreme out-of-pocket costs could also be reduced by regulatory incentives and constraints within the Medicare system. Noting the constitutional need to avoid civil conscription, options could include removal of access to Medicare rebates for a service if the fee is extreme; introducing incentives, similar to GP bulk-billing incentives, that reward medical specialists for charging certain patient populations specified fees; or through more radical measures, such as making Medicare an opt-in system for doctors who agree to meet particular charging guidelines.
Variations in fees charged by particular medical and surgical specialist groups can generate barriers for patients. History suggests self-regulation is unlikely to change this situation, and increasing MBS rebates will have little effect. While the increasing numbers of specialists should put downward pressure on prices, the scale of this pressure is difficult to predict. If the government wants to improve the affordability of medical specialist services without incurring large, uncontrollable costs, direct regulation is one option, but one that is likely to generate considerable debate. Improving the transparency of pricing could increase competition and place downward pressure on unreasonable fee-setting, and may provide the most affordable and fair approach to the problem.
Holistic medicine provision in the outback
Overcoming the barriers to chronic disease management in rural areas
The Royal Flying Doctor Service (RFDS) has been providing essential medical services to rural and remote Australia since its inception in 1927. The service, founded by Reverend John Flynn, started as a single base at Cloncurry in Queensland1 and now operates out of 21 bases, providing both primary care clinics and emergency retrieval services. RFDS has been servicing clinics from its Broken Hill base since the 1940s; by 1970, there were three full-time doctors conducting the clinics and running the on-call service via the radio network. In 2016, Broken Hill doctors treated patients in 17 different clinic locations each month. On most weekdays, there are general practitioners at three clinic sites, along with dentists, nurses and mental health practitioners.
RFDS is well recognised within Australia and internationally as the only provider of emergency care to large swathes of the outback. Television shows, such as The Flying Doctors and Outback ER, make acute care and cutting-edge medicine familiar to the public. What is less well known, however, is the organisation’s extensive involvement in delivering primary care services to people living in remote locations.
Chronic disease management (CDM) is a key component of the primary care services offered, and the appointment of a practice nurse in 2011 was the first step taken to focus on CDM. The nurse’s initial task was to create and manage the chronic disease register. This formalised the disease database and enabled the setting up of recalls to ensure that patients received regular follow-ups, and it also required doctors to comply with the full functionality of the MedicalDirector system. In addition, the opening of the Clive Bishop Medical Centre (CBMC) at the airport base, in August 2014, allows patients from remote locations to see an RFDS doctor when they are in town in between remote clinic days. Thus, in recent years, the RFDS has redefined its role from bush clinics and emergency evacuations to include a more comprehensive primary care approach in an effort to improve chronic disease outcomes.2 However, due to a number of factors, RFDS is still limited in its ability to deliver high quality primary care.
This model of care in remote New South Wales requires significant investment in medical staff. In an area of 640 000 km2 and with about 6000 patients who live outside Broken Hill,3 eight whole-time-equivalent (WTE) GPs are needed to provide appropriate primary care and emergency services — an increase from four WTEs in 2000. Staff fatigue management and CDM considerations have driven the increase in staff numbers. Clinical, pilot and engineering staff salaries, along with aviation fuel, are all required to enable aircraft-serviced clinics and incur a high cost per patient.
In a metropolitan setting, a patient may need an emergency ambulance ride to hospital at a cost of $364 plus $3.29 per km travelled.4 However, in remote locations an $8 million dollar aircraft will need to be sent out at a cost of about $3000 per hour flown. Although the secondary care costs may be expected to be comparable, the approximate tenfold transport cost impacts significantly on health budgets, and demands optimised local CDM and patient concordance.5,6
Western countries have resourced primary care significantly in the past decade, with GPs incentivised to improve CDM. For example, the United Kingdom Qualities and Outcomes Framework rewards GP practices for the overall control of diabetes, chronic obstructive pulmonary disease and cardiovascular disease.7 In Australia, GP management plans (GPMPs) can be charged at a significant premium ($144.25) compared with a standard long Medicare consultation ($71.70).When done well, this should have the effect of more reliable monitoring and control of chronic conditions.8
The Commonwealth contract for the RFDS South Eastern Section, however, does not place a premium on these services. Standard RFDS consultations outside Broken Hill are funded based on historical data; CBMC consultations are Medicare bulk billed under a separate arrangement. When GPMPs are used in remote settings, they do not enable patients to access allied health services — such as diabetes educators, podiatry or physiotherapy — under the Medicare-funded scheme. Patients must either finance it themselves, or book an appointment in a Medicare billed location to access Team Care Arrangements funding.
By involving its in-house multidisciplinary team (funded by a variety of income streams), including practice nurses, women’s and children’s nurses, mental health practitioners and substance misuse workers, RFDS has sought to develop services in line with current best practice. Additional integrated team-based care with medical (generalist and specialist), nursing and allied health staff is known to be associated with improved health outcomes in patients with chronic illnesses.9 Rural and remote primary care centres, such as clinics in far west NSW, are less likely to have a team approach because of limited access to allied health workers.9,10
Recruitment and retention of doctors
RFDS doctors at Broken Hill are required to have a fellowship of the Royal Australian College of General Practitioners or a fellowship of the Australian College of Rural and Remote Medicine. Attainment of these qualifications ensures the standard of knowledge and training of the GPs responsible for treating patients in remote settings. However, the reality is that a full-time doctor may only conduct 2–3 clinics per week (spending the rest of their time on call). With travel time to clinics of up to 2 hours each way, and the attendance varying from 8 to 16 patients per day, clinical skills are used less often and confidence may diminish. Some doctors find this frustrating; others are happy to have time out of private general practice.
The maintenance of clinical skills in emergency care is more challenging. Practitioners need to complete a regular cycle of courses — including Advanced Paediatric Life Support, Advanced Life Support in Obstetrics, Early Management of Severe Trauma and airway skills updates — but there may be months between course completion and the need to use the skills. The gap between competence and confidence may be too much for some doctors to bear, and for those who prefer the emergency to the routine, there is not enough excitement.
Therefore, there are two competing challenges to address: where to find GPs who are experienced enough in their field to be able to manage well the uncertainty in remote places — whether face to face or over the phone — and enough emergency cases to keep this self-selecting group interested. Then of course, there is the remoteness of the place; 1200 km from Sydney and 500 km from Adelaide is too much for most Australian GPs. At present, the full-time practitioners are UK or Irish graduates, and the longest serving of them has been employed for 3 years.
Continuity of care
If a high staff turnover is not controlled, continuity of care in each clinic site will be adversely affected. Even when fully staffed, manning 17 clinic sites and rostering night shifts means that doctors have to be rotated. However, it has been shown that both patients and doctors prefer to know each other as part of an effective therapeutic relationship.11,12 This is an important factor in the effectiveness of CDM and patient engagement.
In addition to the RFDS doctors, health services in Wilcannia, Ivanhoe and Menindee are simultaneously provided by GPs from Maari Ma Health, which is the Indigenous community controlled health organisation. Five Local Health District (LHD) facilities, which include these three, also provide nursing staff at these sites. With the rotating system of both RFDS and Maari Ma Health rosters, along with significant use of agency nursing in remote sites, it is easy to recognise the fragmentation of what should ideally be integrated care. The responsibility for CDM of certain groups falls between the gaps sometimes, and it is not always clear who should be keeping track of follow-up and recall systems. There is, therefore, room for further system development and collaboration here.
Medical records
GP and hospital records are now multi-user friendly and most sites enable multidisciplinary teams to make entries within the same system. However, Maari Ma Health has a separate MedicalDirector system from RFDS, and LHD has recently upgraded to NSW Health’s latest hospital electronic medical record system. Thus, the usual norms of primary care, in which a GP is confident that the electronic record is complete, have not been possible to achieve in recent years. RFDS doctors are fully oriented to the need of keeping updated records for emergency and primary care consults, so that colleagues may be apprised of their decision making.
Social considerations
In remote NSW, there are many circumstances that may impact the clinical follow-up of medical conditions. It is widely believed that logistical, economic and cultural factors affect the low attendance rates for CDM in remote settings.13,14 The reasons for low RFDS clinic attendance rates include socio-economic conditions, the lower likelihood that males in rural communities will use preventive health services than urban males, and a higher proportion of Indigenous people.14 Logistical dimensions of proximity, affordability, accommodation, timeliness and psychosocial attitudes and beliefs are well known to hinder continued primary care in remote regions.13–15 Identifying infrequent users of primary health care who have chronic disease, with consideration of culturally appropriate preventive care, will assist in targeting those patients who require medical services.
There are still many barriers to the high quality management of chronic conditions. Efforts to improve this situation should focus on enhancing continuity of care, follow-up systems and planning of a team care approach. The increased use of telehealth technologies will be an important part of remote consultations, and current initiatives to improve CDM are of the utmost importance.
Variation in the costs of surgery: seeking value
Transparency is key to achieving affordability of health care
There is increasing concern about the sustainability of health care in Organisation for Economic Co-operation and Development (OECD) countries. Australia currently spends US$6140 per capita — or 9.1% of its gross domestic product — on health care.1 Moreover, there is evidence that health care costs, including out-of-pocket (OOP) expenses, are rising.2 In Australia, 68% of health care costs are funded through the public health system, with 32% from other sources, including private health insurers and OOP expenses.2 To encourage Australians to take out health insurance, the private health system is subsidised by a private health insurance rebate, which costs the public about $5 billion per year.2 Private health insurers derive their income from premiums, which have risen an average of just under 6% per year since 2012, well above the inflation rate or the consumer price index.3 Individual OOP expenses are also rising at an average rate of 6.2%; they have more than doubled in a decade and accounted for 17.8% of Australia’s $140 billion health care spending in 2013–14.2
Value is defined as the health outcomes achieved per dollar spent.4 Moreover, data on performance and outcomes are fundamental to the ability to determine value. Reports on variation across the country,5 including comparison with other OECD countries,6 may prompt a review of activity (the treatments and procedures the health system provides), but cannot determine their value without measurement of costs and outcomes.
To ensure high value, the procedures performed must be appropriately indicated, avoiding overservicing or selecting a particular treatment when its likelihood of success, compared with the alternatives, is limited. Health professionals have an ethical responsibility to avoid waste in health care — not only by better targeting resources, but also because “useless tests and treatments cause harm”.7
The interim report on the Medicare Benefits Schedule (MBS) review8 highlights a recurring theme of how to better inform the system through stakeholder feedback based on contemporaneous data. There is currently limited use of health care data, a lack of meaningful clinical reports and often a failure to engage clinicians in clinical governance. This includes costs and fees charged across the health system and informing consumer choice.
The Royal Australasian College of Surgeons (RACS) and Medibank, Australia’s largest private health insurer, have published reports on surgical variance, which detail the cost and outcomes of care in selected high volume procedures for general surgery, otolaryngology, urology, orthopaedics and vascular surgery.9
Data sources and analysis
The data in the reports were extracted from administrative claims — received by Medibank from private hospitals and specialists — for treatment provided to Medibank policy holders. This initial analysis looked at hospital separations with an admission date in 2014 and any follow-up hospital separation funded by Medibank within 6 months of discharge. The reports were compiled from hospital claims data, hospital casemix protocol data and MBS data relating to diagnoses, interventions and patient demographics. We analysed service use, including transfers to intensive care units (ICUs) and rehabilitation, and plotted outcomes based on length of stay, hospital acquired complications, re-admissions and re-operations. The data on costs included the Medicare item numbers billed, total costs of a hospital admission, cost of prostheses, and OOP expenses.
Procedures were identified by MBS code and selected on the basis of volume to ensure a sufficient spread across surgeons performing them. A principal procedure was identified for each hospital separation, and for most of them, this was the highest value MBS item fee from the medical claim. Where multiple MBS codes described a single procedure, the similar MBS codes were combined. Surgeon level analysis was limited to those with at least five procedures in the dataset. Medibank did not share the identity of the individual surgeon.
Hip replacement as an example
The RACS and Medibank reports on clinical variation focus on specific surgical procedures without risk adjustment.9 For patients who had hip or knee arthroplasty, there was wide variation in the length of stay, use of ICU bed days or rates of transfer to inpatient rehabilitation.
We have used hip replacement as an example in our discussion of costs, fees and value. The rates of hospital acquired complications were reassuringly low.
The cost of a surgical episode of care includes the sum of hospital, surgeon and other providers’ fees, the prosthesis, and pharmaceuticals. The use of ICU after the operation or transfer to rehabilitation following a surgical admission varied considerably. For the 299 surgeons who performed at least five procedures, the average separation cost of a surgeon ranged between $18 309 and $61 699 with a median of $26 661 (the average total cost per hospital separation was $27 310). High volume surgeons showed greater congruity and were closer to the median in terms of the overall cost of a hip replacement. There was little variation in regional (state and territory) total costs.9
Prices for hip prostheses varied from $4908 and $16 178, with a median of $10 727 (Box 1). In Australia, there is considerable disparity in the cost of prostheses when prices are compared between the public and private health systems.10 The amount paid by private health insurers for a prosthesis in the private sector can be twice the amount in public hospitals. Australia also pays a high price for prostheses when compared with other OECD countries.10 The government has recently announced a reduction in the price to be paid for items on the Prostheses List, reducing a hip prosthesis by 7.5%.11
OOP expenses
Medical practitioners in Australia, including surgeons, often augment the fee paid by Medicare and the private health insurer with a copayment paid by the patient. This is known as an OOP charge and is often resented by patients with private health insurance. There is considerable variability in the rebate a health fund will reimburse with regards to a provider’s fee and, therefore, some difference in gap payments is to be expected.
Medibank-insured patients who had a hip replacement incurred an OOP charge by the principal surgeon in 39% of separations, and the average OOP fee was $1778.9
For the 299 surgeons who performed at least five hip replacements, 142 (47%) did not charge any OOP. The average OOP charged ranged from none to $4057.9 The OOP surgical fee is a large but not the only component of gaps paid by patients. OOP charges for other medical services, including charges raised by the anaesthetist, assistant surgeon, and for diagnostics, were charged in 80% of the hospital separations, with an average charge per patient of $342.9
What is a reasonable fee?
The RACS recognises — as does the Australian Medical Association — that gaps are necessary and that, apart from the variability in insurance coverage, the underlying cause of gap fees is the failure of government reimbursement through Medicare to keep pace with inflation and the cost of providing a service.12 The RACS view is that the fees charged should be reasonable and in line with the skill, effort and risks associated with performing a procedure and providing perioperative care.13
Surgeons should not take advantage of the vulnerability of their patients. No one should need to access their superannuation, remortgage their home or resort to crowd funding to have surgery that is clinically indicated. In Australia, the public system is always available for emergency and urgent surgery. RACS has made explicit statements in this regard, published position statements12,13 and provided advice to patients. The RACS code of conduct14 makes clear what is expected of surgeons in setting and informing patients about fees.
There was also no correlation between the size of the fee charged and the quality of the surgery. Indeed, an unpublished analysis by the RACS and Medibank found that surgical fees charged were not correlated with the length of stay for any procedures studied (Box 2). Length of stay is a reasonable surrogate for quality in well defined, largely standardised unicomponent elective operations, such as joint replacement, which have well established post-operative and discharge protocols.
There is an opportunity to further reduce the length of stay after a joint replacement when comparing the Medibank Private results with the public sector in Victoria15 and Scotland.16 This reduction may be achieved without diminishing the quality; a shorter stay may make savings and increase in value.
Conclusion
The discussion around the affordability of health care must continue. As key members of the health care team, surgeons cannot ignore the total costs of surgical services, the components within that service or the fees associated with their individual activity. The Medibank and other administrative datasets inform us on variation in the components of value — clinical activity, outcomes, reimbursements and costs. Making the reports publicly available9 provides assurance of transparency and accountability and may better inform surgeons’ and patients’ choices in the future.
Box 1 –
Average prosthetic cost for hip replacements
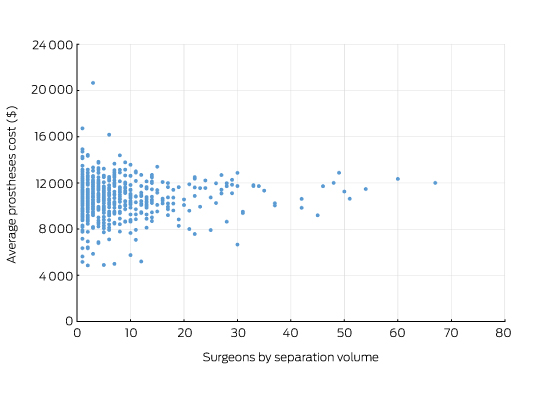
Source: Royal Australasian College of Surgeons and Medibank Surgical Variance Report for Orthopaedic Surgery.9 The data cover the claims paid by Medibank for Medibank policy holders during 2014. Separations that included either Medicare Benefits Schedule items 49318 or 49321 were recorded as the highest value procedural item on the medical claim.
Box 2 –
Total knee replacement: plot of the out-of-pocket fee charged by the surgeon and hospital length of stay showing no correlation
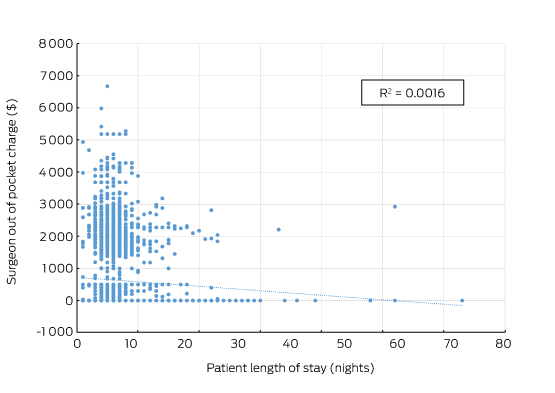
Source: Royal Australasian College of Surgeons and Medibank. The data cover the claims paid by Medibank for Medibank policy holders during 2014. Separations that included either Medicare Benefits Schedule items 49518 or 49521 were recorded as the highest value procedural item on the medical claim.
Overcoming negative perceptions among Australian medical students about a career in general practice
Encouraging medical students to pursue a career in general practice is a global problem with an Australian solution
General practice is the cornerstone of the Australian health care system, and a critical component of health care systems around the world. However, recruiting a general practice workforce capable of meeting community needs remains a global challenge.
Canada is experiencing a critical shortage of general practitioners, with 14.9% of the population without a GP in 2014.1 Similarly, the United Kingdom faces a severe shortage of GPs, coupled with insufficient numbers of medical students choosing general practice as a career.2 The number of applications for GP training in the UK fell between 2013 and 2015, with 12.4% of training posts unfilled in 2015.3 In the United States, only 11.7% of 2016 residency training positions were for general practice, and 155 places were left unfilled.4 Further, an international study found that general practice is poorly perceived by medical students, with students across seven countries indicating that they were less interested in the specialty, perceiving general practice as less intellectually challenging, with lower prestige and poor remuneration.5
In 2007, Australia was facing a similar situation. Negative perceptions of general practice among medical students were a barrier to overcoming a looming GP shortage6 and graduates were increasingly choosing to not pursue the specialty as a career.7 In 2005, only 532 of 600 available GP training positions were filled.6 An ageing workforce6 and medical students’ lack of interest in general practice presented a challenge for policy makers — how could this negative perception be overcome?
Creating the General Practice Students Network
In 2005, Joe Rotella, a Melbourne medical student, recognised the negativity about general practice in Australian medical schools. With the support of General Practice Registrars Australia (GPRA) and a successful funding application to the federal government, he developed the concept of a network of student clubs to reverse this negativity.
Today, General Practice Students Network (GPSN) clubs exist in each Australian medical school. They are run by student volunteers who organise educational events to promote general practice to medical students, including educational talks from local GPs, career networking nights, clinical skills workshops and rural and Indigenous health events. Each event is planned and presented by students and supported by local organisations, including other student clubs, regional training providers and academic staff at each university. The national council of local clubs is overseen by the GPSN National Executive, a team of students who advocate on behalf of the organisation and oversee the running of the network, with support from staff at GPRA.
Between 2007 and 2016, the membership of the GPSN grew from 121 to 14 199 student members. This rapid growth led to the addition of two new programs:
-
the Going Places Network (GPN), which promotes general practice to prevocational doctors in training hospitals and currently has 3500 junior doctor members; and
-
the John Murtagh First Wave Scholarship, a program that provides placements in general practice for preclinical medical students.
Collectively, the GPSN, GPN and John Murtagh First Wave Scholarship are known as GP First, a pipeline that promotes general practice from the first day of medical school until the commencement of specialty training.
GP First
The strategy of GP First has been to target directly the factors that are known to increase interest in general practice. Enthusiasm for, and commitment to, general practice is an important determinant of whether students will pursue it as a career path.5 Since its inception, the GPSN has worked to foster this enthusiasm by using a peer-to-peer model that takes advantage of the known positive influence peers can have on student’s perceptions of the specialty.5,8 In 2014, the 21 local GPSN clubs ran 98 events which were attended by 7259 students. Research has also shown that positive role models influence student perceptions of general practice,5 and GPSN events have provided opportunities for students to network with GP registrars who are seen by students as the most current and accurate source of career information.8
Positive exposure to general practice has also been found to improve student perceptions of general practice,5,9,10 and since 2008, the John Murtagh First Wave Scholarship has provided general practice placements for over 600 medical students. Of these students, more than 92% found the program extremely useful in helping them with their future career choice and more than 77% said the program made them more likely to consider general practice as a career.11
Since the GPSN was founded, there has been a significant shift in the GP training landscape. In 2005, the Australian General Practice Training (AGPT) program was only able to fill 532 of its 600 training places. Only 366 of these applicants (69%) were Australian medical school graduates,12 representing 24.4% of the 1503 medical students who had graduated the previous year.13
In 2014, there were 2026 applications for 1500 AGPT training places; 1421 of these were graduates from Australian medical schools,14 representing 41.3% of the 3441 students who had graduated from Australian medical schools in the previous year.13 This represents not only an increase in the number of applications in absolute terms, but also substantial growth in the percentage of graduates pursuing general practice training.
Between 2011 and 2013, GPRA worked with General Practice Education and Training (GPET) to quantify the success of the GP First program by tracking the number of AGPT applicants who were either First Wave scholars or members of the GPSN and GPN. The percentage of applicants from GP First increased from 11% in 2011 to 25.6% in 2012 and in 2013, 35% of acceptances into training were from GP First.11
The more than doubling of graduating medical students over the 10 years has undoubtedly contributed to the increase in GP training applicants. However, there is little recent research quantifying the impact of other contributors to this increase. A 2011 study found that factors contributing to choice of career for GP registrars included the quality of undergraduate general practice placements, exposure to GP role models, awareness of AGPT and the GP colleges at the student level, as well as the flexibility of GP training.12
During the time that the GPSN program has operated in Australian medical schools, there has been a significant improvement in medical students’ perception of general practice since its low popularity in 2005. From 2010 to 2013 the percentage of graduates identifying general practice as their top choice for future medical specialty increased from 12.3% to 17%.15 Indeed, general practice topped the list in 2013, placing higher than internal medicine (16.6%) and surgery (16%).15
GP First is undoubtedly only one of a number of factors that may have helped to improve the perception of general practice among medical students and, unfortunately, the impacts of its three programs for students have not been quantified. With the increase in applicants for GP training, the federal government no longer sees a need for a program to promote general practice to medical students. In December 2015, the government cut all funding for the GPSN, the GPN and the John Murtagh First Wave Scholarship.
The future
The focus of GPSN clubs across Australia has shifted from promoting general practice to supporting the future leaders of the specialty. Local events and projects from the four national working groups will continue to focus on areas of need in the community, including rural health and Indigenous health, while also working on closing gaps in general practice education for medical students and junior doctors. The John Murtagh First Wave Scholarship will survive in a reduced form, supported by corporate sponsorship, to ensure that future medical students continue to have positive experiences of general practice.
In less than 10 years, the GPSN has grown from one medical student’s idea into a successful national organisation run by hundreds of volunteers who organise events attended by thousands of students each year. With the loss of government funding, GPSN clubs now face the challenge of securing their own survival while continuing to run events that inspire the next generation of GPs and ensure students are equipped to navigate the changing landscape of primary health care in Australia.
Australia has reversed the downward trend in GP training numbers seen around world, with the demand for places now exceeding supply. The GPSN is just one of several possible contributors to medical students’ increased interest in general practice. Research is needed to identify and quantify the impact of the various demographic factors and workforce programs that are contributing to this change, so that other nations can learn from Australia’s success in securing our future primary care workforce.
Daily step count and the need for hospital care in subsequent years in a community-based sample of older Australians
The known Most investigations of the benefits of physical activity for health have used self-reported measures of physical activity of limited validity. As a large proportion of people admitted to Australian hospitals are over 55, quantifying the factors that influence their need for hospital care is important.
The new An increase in step count from 4500 to 8800 steps per day was associated with 0.36 fewer hospital bed-days per person per year.
The implications Health interventions and urban design features that encourage walking could have a substantial effect on the need for hospital care, and should be features of health policy.
Epidemiologic evidence strongly suggests that increased levels of physical activity are associated with the reduced incidence, prevalence and mortality of a range of diseases.1 Its impact on the use of health services, however, is not well studied. With an ageing and largely inactive population putting increased pressure on inpatient hospital services, it is important to understand the extent to which increased physical activity might reduce the number of hospital admissions. The direct health care costs for Australia of physical inactivity were estimated to be $377 million in 2000, but this figure was based on population-related risks for a range of diseases, rather than assessment of the actual use of services.2
Data on hospital admissions for particular diseases are available; for example, for patients in Denmark with chronic obstructive pulmonary disease the risk of hospital admission over the subsequent 12 years was 28% lower for those who reported any physical activity than for patients who reported no activity.3 In another study, the risk of hospital admission for women with venous thrombo-embolism was 24% lower in those walking more than 5 hours a week than for those who walked less than one hour per week.4
Health care costs have also been studied; for example, in a large community-based sample of Canadians over 65 years of age, the self-reported health care costs for people who met physical activity guidelines were CAN$1214 (60%) lower over the following 12 months than for those who were inactive.5 Similar results were found in Japan, with 12-month health care costs £174 (13%) lower for those walking one hour a day than for people who did not.6 Contrasting findings were made in the younger National Health and Nutrition Examination Survey (NHANES) sample in the United States (average age, 45 years), for whom there was no association between self-reported physical activity and total health expenditure in the following year.5,7
Only one previous study has investigated associations between objectively measured physical activity and subsequent health service use. A United Kingdom cohort of 240 people (average age, 78 years) wore accelerometers for a week and was followed for an average of almost 5 years. The incidence of unplanned hospitalisation was 2.13 times higher in those with low levels than in people with higher daily levels of moderate to vigorous physical activity (mean, 3 v 39 minutes), and 1.81 times higher in those with low daily step counts than in people with high counts (mean, 2100 v 7100 steps). There was also an inverse association with the number of prescriptions written, but not for the number of general practice consultations or referrals to specialist care.8 The question of reverse causation was not investigated: the possibility that, rather than physical activity improving health, illness causes people to be less active.
Research into population-wide physical activity has been hampered by the low validity of self-reported measures, so that more recent published research has favoured objective measurement with pedometers, accelerometers, and metabolic methods.
We enrolled people over the age of 55 years in a cohort study during 2004–2007, collecting a wide range of data on baseline variables, including step counts. This was the first cohort of more than 1000 people for whom data on an objective measure of physical activity at baseline were collected, giving us a unique opportunity to examine the effect of baseline step counts on hospital use in subsequent years.
Methods
The Hunter Community Study includes a cohort of community-dwelling men and women aged 55–85 years who reside in Newcastle, New South Wales. Participants were randomly selected from the NSW state electoral roll and contacted between December 2004 and December 2007. Listing on the electoral roll is compulsory in Australia, and it is estimated to be 93.6% complete.9 A modified Dillman recruiting strategy10 was applied. Two letters of introduction and an invitation to participate were posted to the selected candidates; individuals who did not respond to the initial letters were telephoned by a research assistant if a publicly listed number was available. If contact was not established after five attempts, the individual was classified as a non-responder. People who could not speak English or were living in residential aged care facilities were excluded.
After providing consent, participants were asked to complete two questionnaires and to return them when they attended a data collection clinic, at which a series of clinical assessments was undertaken. Consent from participants was also sought at this time to link personal information obtained during the study with Medicare data (Medicare and Pharmaceutical Benefits Scheme) and local hospital databases. A package of three further questionnaires, to be returned by reply-paid post, was given to participants to complete at home after their clinical assessment. Administering the questionnaires in segments before and after the clinical phase aimed to reduce the burden for respondents and to thereby maximise attendance for clinical assessments.
Step count was recorded over a week by a pedometer (Yamax) worn during waking hours, and participants recorded the daily step count in a diary. In 2004–2007, there was little community awareness of step count targets, and no guidance was given as to how many steps constituted a healthy amount of activity. The pedometer was worn on the waist belt, in line with either leg. Days with less than 9 hours’ wear were excluded from analysis, and a daily average count was calculated for participants with at least 3 days of wear.
Hospital admissions for the 2623 people who consented to record linkage were retrieved from both public and private hospitals. All hospital admissions in NSW are coded by professional hospital coders using the International Classification of Diseases, version 10 (ICD-10), with a first position code for the principal reason for admission and up to ten other codes for comorbidities. We chose bed-days rather than admissions as the outcome measure because it more accurately reflects health care costs, and physical activity could conceivably influence the severity as well as the incidence of disease.
Statistical methods
Baseline demographic information for the analysis population (all those with complete pedometry, hospital admissions and relevant clinical and demographic data) was summarised as means and standard deviations for continuous variables and as frequencies and percentages for categorical variables. The distributions of these variables were compared with the NSW 2006 census population aged 55–85 years (where available) in χ2 tests.
For each consenting participant, we estimated the number of hospital bed-days from enrolment in the study to 31 March 2015, based on data in the admitted patient database. The number of days in hospital was defined according to the admission and discharge dates, initially for all admissions, and then stratified by ICD-10 and procedural codes, allowing us to determine cancer-, diabetes- and cardiovascular disease (CVD)-specific hospital separations. Private hospital follow-up time was excluded for CVD-related admissions because data were missing for some years.
We initially analysed the total number of bed-days after recruitment, and then performed a sensitivity analysis that excluded data for the first 2 years of follow-up, in order to examine the effect of reverse causality.
To determine the appropriate set of confounding variables for estimating the effect of physical activity on the number of hospital admissions, we constructed a directed acyclic graph (online Appendix). The following variables were deemed sufficient: age at the baseline survey, sex, number of medications, number of comorbidities (angina, asthma, heart attack, osteo-arthritis, rheumatoid arthritis, stroke, diabetes, elevated cholesterol levels, thyroid conditions, depression or anxiety, atrial fibrillation, bronchitis or emphysema, cancer), smoking status (never, former or current smoker), level of alcohol consumption (never drink, safe, moderate, hazardous/binge, hazardous/chronic, unknown), and most advanced level of education.
A negative binomial regression model was used, with number of hospital bed-days as the outcome, log length of follow-up as an offset, and step counts, education level, sex, number of comorbidities, number of medications, smoking status, and alcohol consumption included as independent variables. In the models for disease-specific bed-days, the number of participants with no bed-days was high, so a zero-inflated negative binomial model was employed for these analyses. Incidence rate ratios are presented with Wald 95% confidence intervals [CIs], P values, and least squares mean estimates for number of bed-days at the lower quartile, median, and upper quartile boundaries for step counts. Goodness of fit was assessed by visually inspecting estimates of observed and model-predicted bed-days, and plots of deviance residuals v independent variables. All analyses were conducted in Stata 13.1 (StataCorp).
Ethics approval
Ethics approval was granted by the University of Newcastle Human Research Ethics Committee (reference, H-820-0504a).
Results
Invitation letters were sent to 9784 individuals randomly selected from the electoral roll. Of the 7575 subjects who responded, either in person or through a relative, 258 were ineligible (148 did not speak English, 92 had subsequently died, 18 had moved to an aged care institution); 3440 refused and 3877 agreed to participate. A total of 3253 people actually participated, giving a response rate of 44.5% of eligible responders. The participant group reflected the Australian population aged 55–85 years in terms of sex and marital status, but was slightly younger. Further details of recruitment and the representativeness of the sample have been published elsewhere.11
Valid step count data were recorded for 2458 participants, of whom 2174 had consented to record linkage. The baseline number of medications was not well reported (20% missing data), so this variable was omitted from further analyses. We present a complete case analysis of the data for the 2110 participants for whom complete data on all variables of interest were available (Box 1).
Compared with the NSW population aged 55–85 years in 2006, the proportion of participants who did not drink alcohol was similar; more had a university or trade qualification, fewer were smokers, and there was a statistically significant different age distribution.
The total number of person-years followed up was 17 374 (excluding the first 2 years, 13 514 person-years), with a mean of 8.2 years (range, 7.0–11.1 years). The median daily step count was 6.6k steps (k = 1000; interquartile range, [IQR], 4.5k–8.8k steps); 1% of participants had step counts greater than 16.4k, and the maximum value was 23.9k. The median overall time in hospital was 2 days (IQR, 0–9 days); 1% of participants spent more than 135 days in hospital, and the maximum stay was 384 days. Excluding the first 2 years of follow-up, the median time in hospital was 0 days (IQR, 0–5 days; maximum, 294 days). The number of bed-days of hospital care associated with particular diseases is shown in Box 2.
Association between step count and number of bed-days
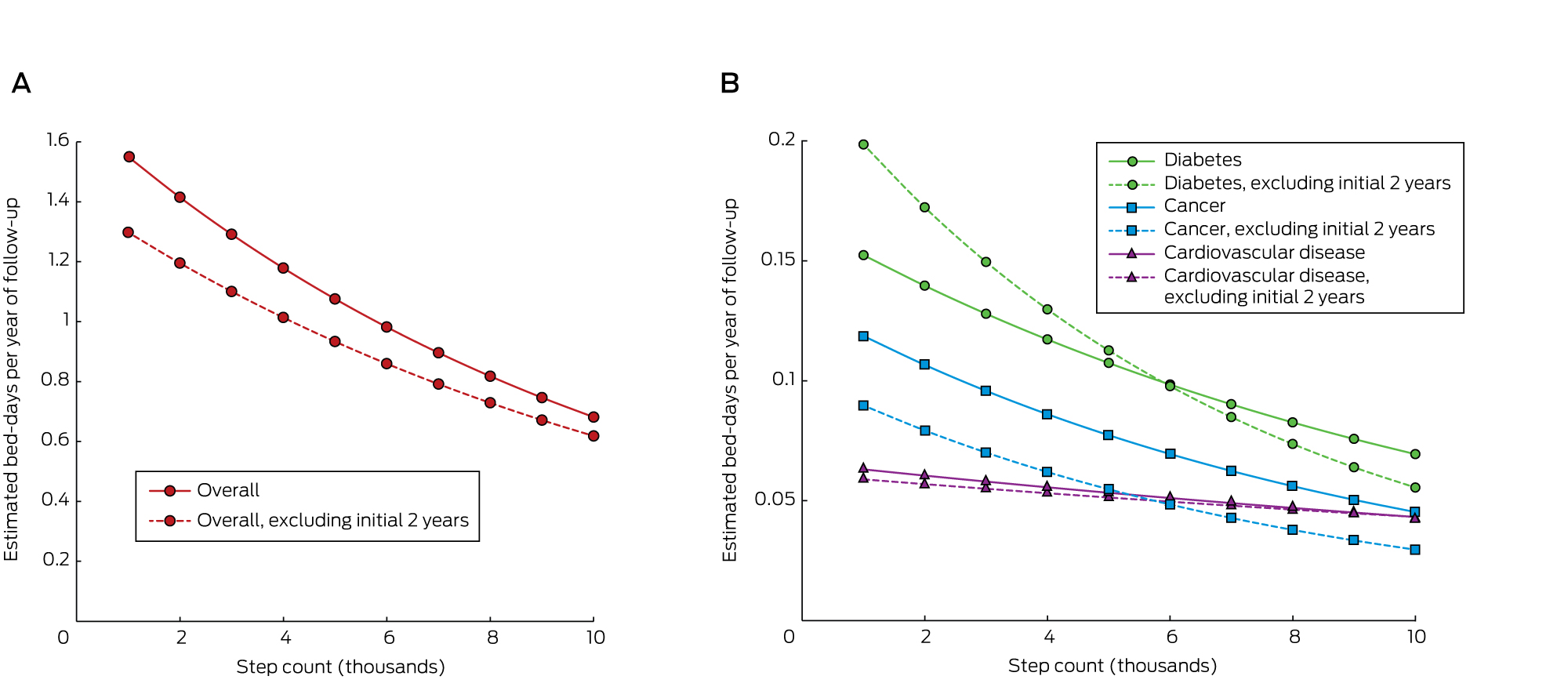
The average number of bed-days per year for people at the 25th percentile step count was 1.12, for those at the 75th percentile it was 0.76, a difference of 0.36 bed-days, or 32%. After excluding the first 2 years’ follow-up, the average number of bed-days per year for people at the 25th percentile step count was 0.97, while for those at the 75th percentile it was 0.68, a 30% reduction (Box 3).
After adjusting for potential confounders, the overall estimated number of bed-days per year of follow-up decreased by 9% for each 1000-step increase in daily step count (95% CI, –10% to –6%; P < 0.001). A higher step count was found to be associated with fewer bed-days for cancer and diabetes, but not for CVD (Box 3). Estimated bed-days for step counts in the range 1k–10k are shown in Box 4.
A sensitivity analysis was conducted to explore the effect of outliers. Models were re-run after exclusion of the top 1% of values for bed-days and step counts. The results were essentially similar, but the benefit for patients with cancer was no longer statistically significant (data not shown).
Discussion
We report the first investigation of the association between objectively measured physical activity and hospital use over an extended follow-up period. People taking 8800 steps per day (the 75th percentile boundary) spent almost one-third of a day less in hospital per year of follow-up than people taking 4500 steps per day (the 25th percentile boundary). The estimated difference was slightly smaller (0.29 days) when the first 2 years of follow-up were excluded, and we regard this smaller figure as the best estimate of the causal effect. This difference equates to a 30% lower requirement for hospital care being associated with 4300 extra steps per day, or about 40 minutes of walking.
While the effect of step count on cancer and diabetes admissions data was significant, we were surprised to find no significant effect on CVD admissions; this anomaly may be related to the fact that data for CVD admissions in the private sector were missing.
The strengths of our study included the large community-based sample, our adjustment for appropriate confounders, the extended follow-up, and complete ascertainment of hospital admissions from NSW hospital records. The response rate with respect to recruitment was only 22%, but the sample was nevertheless reasonably representative of the NSW population. Potential weaknesses included the possibility of residual reverse causality, even after removing the first 2 years’ follow-up and adjusting for the number of diagnoses at baseline. Our analysis assumes that the week of step counts recorded at baseline was typical for the participant’s usual activity. There is also an inherent limitation in the use of step counters to record overall physical activity, as they do not capture, for instance, swimming or cycling, nor do they record the intensity of activity. They do, however, capture all movement throughout the day, and we have previously shown that step counts have greater validity than a self-reported physical activity scale.12
The cost of a day in hospital in Australia in 2012–13 was $1895,13 so $550 can potentially be saved annually for each person who increases their physical activity by an achievable 4300 steps per day. These steps can be accumulated as many brief activities throughout the day, or as steady walking for about 3 kilometres. Previous investigation of the dose–response curves for various health indicators in older people has shown that the steepest part of the curve is at the lower end of activity.14 Moving from 3000 to 5000 steps per day is of greater benefit than moving from 8000 to 10 000 steps.
Health implications
Our estimates of the hospital care burden associated with varying levels of physical activity suggest that large reductions in hospital use may be possible with measures that increase community physical activity levels, such as health coaching, restricting parking availability, and better urban design.
Box 1 –
Baseline characteristics for the 2110 participants, compared with those of the standard New South Wales population aged 55–85 years (2006)
|
Characteristic |
Participants |
NSW population* |
P (χ2) |
||||||||||||
|
|
|||||||||||||||
|
Age (years), mean (SD) |
66.1 (7.4) |
|
|
||||||||||||
|
Age group |
|
|
< 0.001 |
||||||||||||
|
55–59 years |
472 (22.4%) |
26.6% |
|
||||||||||||
|
60–64 years |
541 (25.6%) |
21.0% |
|
||||||||||||
|
65–69 years |
436 (20.7%) |
16.8% |
|
||||||||||||
|
70–74 years |
317 (15.0%) |
13.9% |
|
||||||||||||
|
75–79 years |
229 (10.9%) |
12.4% |
|
||||||||||||
|
80–84 years |
115 (5.5%) |
9.3% |
|
||||||||||||
|
Sex (men) |
1026 (48.6%) |
47.5% |
0.31 |
||||||||||||
|
Medications, median number (IQR) |
3 (2–5) |
|
|
||||||||||||
|
Comorbidities, median number† (IQR) |
2 (1–3) |
|
|
||||||||||||
|
Smoker |
|
|
|
||||||||||||
|
Never |
1170 (55.5%) |
|
|
||||||||||||
|
Former |
805 (38.2%) |
|
|
||||||||||||
|
Current |
135 (6.4%) |
10.9% |
< 0.001 |
||||||||||||
|
Alcohol consumption |
|
|
|
||||||||||||
|
Teetotaller |
590 (28.0%) |
29.7% |
0.088 |
||||||||||||
|
Safe drinker |
979 (46.4%) |
|
|
||||||||||||
|
Moderate drinker |
151 (7.2%) |
|
|
||||||||||||
|
Hazardous drinker/binge |
124 (5.9%) |
|
|
||||||||||||
|
Hazardous drinker/chronic |
96 (4.6%) |
|
|
||||||||||||
|
Unknown |
170 (8.1%) |
|
|
||||||||||||
|
Education level |
|
|
|
||||||||||||
|
Primary schooling only |
47 (2.2%) |
|
|
||||||||||||
|
Secondary schooling completed |
493 (23.3%) |
|
|
||||||||||||
|
Secondary schooling not completed |
399 (18.9%) |
18.4% |
0.55 |
||||||||||||
|
Trade or TAFE qualification |
558 (26.5%) |
21.5% |
< 0.001 |
||||||||||||
|
University or other tertiary study |
490 (23.2%) |
10.5% |
< 0.001 |
||||||||||||
|
Other or not applicable |
123 (5.8%) |
|
|
||||||||||||
|
|
|||||||||||||||
|
TAFE = Technical and Further Education college. * Data generated in TableBuilder (http://www.abs.gov.au/websitedbs/censushome.nsf/home/tablebuilder). † Of the 13 conditions listed in the Methods. |
|||||||||||||||
Box 2 –
Number of hospital bed-days for the 2110 participants, and proportions of bed-days associated with cardiovascular disease, cancer and diabetes
|
|
Number of bed-days |
Proportion |
|||||||||||||
|
|
|||||||||||||||
|
Overall |
|
|
|||||||||||||
|
All follow-up |
28 876 |
|
|||||||||||||
|
Excluding initial 2 years |
20 172 |
|
|||||||||||||
|
Cardiovascular disease |
|
|
|||||||||||||
|
All follow-up |
1747 |
6.1% |
|||||||||||||
|
Excluding initial 2 years |
1353 |
6.7% |
|||||||||||||
|
Cancer |
|
|
|||||||||||||
|
All follow-up |
1794 |
6.2% |
|||||||||||||
|
Excluding initial 2 years |
1174 |
5.8% |
|||||||||||||
|
Diabetes |
|
|
|||||||||||||
|
All follow-up |
5528 |
19.1% |
|||||||||||||
|
Excluding initial 2 years |
4107 |
20.4% |
|||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 3 –
Association of step count with number of bed-days, and estimated bed-days per year of follow-up (least squares means) for specific step count levels*
|
|
Incidence rate ratio (95% CI) per extra 1k steps |
P |
Estimated bed-days per year of follow-up (95% CI) |
||||||||||||
|
Q14.5k steps |
Median6.6k steps |
Q38.8k steps |
|||||||||||||
|
|
|||||||||||||||
|
Overall |
|
|
|
|
|
||||||||||
|
All follow-up |
0.91 (0.90–0.94) |
< 0.001 |
1.12 (1.00–1.30) |
0.93 (0.84–1.00) |
0.76 (0.68–0.85) |
||||||||||
|
Excluding initial 2 years |
0.92 (0.90–0.95) |
< 0.001 |
0.97 (0.84–1.10) |
0.82 (0.72–0.91) |
0.68 (0.60–0.80) |
||||||||||
|
Cardiovascular disease |
|
|
|
|
|
||||||||||
|
All follow-up |
0.96 (0.91–1.00) |
0.096 |
0.054 (0.04–0.07) |
0.05 (0.04–0.06) |
0.045 (0.03–0.06) |
||||||||||
|
Excluding initial 2 years |
0.97 (0.91–1.03) |
0.27 |
0.052 (0.03–0.07) |
0.049 (0.03–0.07) |
0.045 (0.03–0.06) |
||||||||||
|
Cancer |
|
|
|
|
|
||||||||||
|
All follow-up |
0.90 (0.83–0.98) |
0.012 |
0.082 (0.05–0.11) |
0.065 (0.04–0.09) |
0.05 (0.03–0.07) |
||||||||||
|
Excluding initial 2 years |
0.88 (0.80–0.96) |
0.005 |
0.058 (0.03–0.08) |
0.045 (0.03–0.06) |
0.034 (0.02–0.05) |
||||||||||
|
Diabetes |
|
|
|
|
|
||||||||||
|
All follow-up |
0.92 (0.86–0.98) |
0.013 |
0.11 (0.07–0.15) |
0.09 (0.06–0.13) |
0.08 (0.04–0.11) |
||||||||||
|
Excluding initial 2 years |
0.87 (0.80–0.94) |
0.001 |
0.12 (0.07–0.17) |
0.09 (0.05–0.13) |
0.07 (0.03–0.10) |
||||||||||
|
|
|||||||||||||||
|
Q1 = first quartile; Q3 = third quartile. * After correction for age, sex, number of medications, number of comorbidities, smoking and alcohol status, and education level. |
|||||||||||||||
Is wearable technology an activity motivator, or a fad that wears thin?
Activity monitors may be useful for encouraging healthier lifestyles in people of all ages
In this issue of the MJA, Ewald and his co-authors report on the association between increases in daily step counts and the reduced need for hospital care among older Australians.1 Their findings confirm something international experts widely acknowledge: increasing population levels of physical activity is critical for reducing the global burden of diseases such as coronary heart disease, type 2 diabetes, and breast and colon cancers.2 With fewer than 50% of Australian adults meeting current physical activity guidelines of at least 150 minutes of moderate intensity activity per week,3 the biggest challenge faced by clinical and public health practitioners is how to increase activity levels in our largely sedentary population.
Wearable technology (eg, activity monitors such as Fitbit, Garmin, iWatch etc.) may provide the motivation needed to increase physical activity, especially among those at risk of hospitalisation. Twenty per cent of the Australian adult population (10% of those aged 65 or more) own some form of wearable technology.4 The popularity, mass market appeal, pervasiveness, and widespread availability of wearable devices, combined with their decreasing cost, provide significant opportunities for promoting physical activity in the broader community.
But can these devices increase and maintain physical activity levels in the long term? One-third of American consumers who own wearable technology stop using it after 6 months, but the underlying reasons are poorly understood.5 Did the wearers meet their goals, or did they have trouble using the technology? Most research has focused on device validity and reliability when measuring physical activity, energy expenditure and sleep in younger adult populations; a good level of validity for step measurements has been reported.6 One review that identified 11 studies in which wearable technologies have been used in physical activity interventions reported significant increases in overall activity levels.7
However, the overall effect of such devices on the health and physical activity levels of older adults is largely unknown. Wearable activity monitors are perceived as acceptable and useful by adults aged 70 or more, and older people and those with chronic illnesses are able to use these devices.8 While users may need help with setting up their device and understanding the data it collects,8 initially focusing on step data may help older adults to familiarise themselves with their operation.
Wearable technology provides a ready means for self-monitoring of clinical and behavioural data in real time,7 long regarded by health behaviour change scientists as critical for the adoption (or cessation) of particular behaviours. Researchers have integrated these technologies into different interventions to increase activity levels. A comparison of three intervention strategies (activity monitor, monitor plus cash incentives, monitor plus charity incentives) in 800 Singapore workers found that the activity monitor alone and the monitor plus charity incentives groups were significantly more active (37 and 32 minutes/week respectively) than a control group (no tracker or incentives).9 In contrast, a 24-month randomised control trial in the United States involving 471 adults compared weight loss and changes in body composition, fitness, physical activity, and dietary intake after randomisation to one of two interventions; each included group and telephone counselling, text message prompts, and website study materials. After 12 months, all participants commenced self-monitoring on a website of their diet and physical activity, while the “enhanced intervention” group also received a wearable device. Both groups experienced significant weight loss (3.5 kg and 5.9 kg in the enhanced and standard intervention groups respectively) and increased physical activity levels, but there were no significant differences between the two groups, possibly because both had employed self-monitoring, albeit in different forms.10
Several important messages are currently emerging from physical activity and health research. While individuals will monitor their own activity over time with wearable technology,7 which is important for their adopting a new physical activity or exercise regimen, the devices also have the potential to enhance the likelihood of maintaining increases in physical activity in the longer term. Features that may facilitate sustained improvement include:
-
an accompanying app that enables the wearer to easily track their activity and to receive feedback relevant to set goals;
-
social support from family and health professionals;
-
opportunities to motivate, educate and individually tailor programs;
-
prompts for desirable activity behaviours; and
-
the ability to track other health behaviours and outcomes (eg, diet, weight, heart rate).
Further investigation of wearable technology is needed, particularly in different population groups, with the aim of identifying the key factors for enhancing sustained changes in physical activity. We need to identify how these devices can be integrated into clinical practice in order to improve health outcomes. But for health practitioners with sedentary patients looking for assistance with becoming more active, a wearable activity monitor would be a good first step.
Auditing and standardising care for young people with diabetes: beginning a process to improve outcomes
Clear goals, uniform practices, and expectations influence outcomes for young people with diabetes
Diabetes mellitus is a state of dysregulated metabolism caused by varying degrees of insulin deficiency, compounded by resistance to its metabolic actions. In type 1 diabetes, the most common form in children and adolescents, severe insulin deficiency caused by an autoimmune process triggered by environmental factors ultimately leads to near total loss of insulin secretion, necessitating its replacement. Type 2 diabetes, associated with insulin resistance, obesity and genetic or acquired defects in insulin secretion, affects only 5–10% of children and adolescents with diabetes.1 Hyperglycaemia is the most convenient feature to check in patients, by measuring blood glucose levels several times each day; HbA1c levels can be assessed for longer term monitoring, while abnormalities in protein and fat metabolism are checked periodically. The overall costs to any national health service of daily treatment and later complications are staggering: and, as the numbers of people with types 1 and 2 diabetes are increasing worldwide, so too are the costs.2
The micro- and macrovascular complications of diabetes are related to the degree of metabolic control; moreover, stricter metabolic control at the onset of type 1 diabetes imparts long lasting metabolic benefits that delay complications, even if control later deteriorates (“metabolic memory”).3 Avoiding large swings in blood glucose levels in people with near normal average levels also delays the development of complications.4 Strict metabolic control is therefore fostered by the standards of care recommended by national and international societies, including those in Australia.5,6
The consensus is that HbA1c levels in children and adolescents with diabetes should be below 58 mmol/mol and that variations in glucose levels be minimised.4–6 Achieving this goal remains challenging. Success requires three elements: providing insulin in a manner that mimics normal physiology as closely as possible; integrating treatment with age-appropriate nutrition and exercise regimens; and the family and medical team mutually agreeing upon appropriate goals.
The current standard of insulin delivery is multiple daily injections, consisting of one or two basal injections and three or more injections of short-acting insulin to cover meals and snacks. The twice-daily combination of short- and longer acting insulin constitutes the minimal level of care, and is often reserved for patients with special needs.7 Continuous subcutaneous insulin infusion (CSII) by pumps that adjust for changes in blood glucose levels, and continuous glucose level monitoring by implanted subcutaneous sensors can further improve metabolic control; newer technologies permit parental regulation of insulin delivery via pumps by remote control.7 CSII is recommended from the onset of diabetes by some centres, while others suggest instituting it after an initial “honeymoon period”. Each approach requires educating the patient and their family and the assistance of specialist teams; socio-economic factors such as income, family coherence, education, and mutual trust between the family unit and their caregivers also affect outcomes. Even under ideal circumstances, life with type 1 diabetes requires a discipline that few have.
In this issue of the MJA, the Australasian Diabetes Data Network report the first national audit of data on children and adolescents with type 1 diabetes, based on data for the 2015 calendar year from five major Australian paediatric diabetes centres staffed by internationally recognised specialists, encompassing data for more than 3000 people under 18 years of age with type 1 diabetes of at least 12 months’ duration.8 The results provide a snapshot of paediatric diabetes in Australia, demonstrate the feasibility of using nationally collated data for benchmarking, and to identify both areas of success and those that need improvement. Overall, HbA1c level targets were achieved by only 27% of patients. Mean HbA1c levels were lower in children under 6 than in adolescents (14–18 years of age), probably reflecting greater parental control in the younger children. This low overall level of control is of some concern, especially in adolescents, but it reflects how difficult it is to attain targets, and is also consistent with results from other centres.9 Almost one in five children were receiving twice-daily insulin injections; the disparity between the various centres in the prevalence of this approach probably reflects local preferences more than the accommodation of patients’ needs. There were also variations in the adoption of CSII, which appeared to achieve better glycaemic control. One-third of the children were overweight or obese, indicating the need for a national review of nutrition and exercise guidelines.
The studies cited by Phelan and her co-authors,8 as well as more recent reports, indicate that clear goals, uniform practices, and expectations influence outcomes for young people with diabetes.10 Their results point to the need, feasibility and opportunity for harmonising data collection, for identifying deficiencies in treatment, for seeking more resources when appropriate, and for implementing more uniform treatment strategies across centres.

 more_vert
more_vert