A SIXTY-five year-old man was slowly dying in a San Diego hospital. An unstoppable bacterial slime growing on a life-sustaining heart implant was seeding an infection penetrating his sternum, poisoning his blood, and destroying the flesh covering his chest.
Intravenous antibiotics were scarcely keeping the infection at bay. Forty times over 3 years, he was taken to the operating room and anaesthetised while surgeons scraped away dead tissue, vacuumed bacteria-laden material, and sterilised and dressed an open wound that gradually grew to the size of a lemon. Images of his upper body show a 15 cm long vertical wound along the midline of his chest. At the caudal end, a 2 cm black, mushroom-shaped base of a pump – a key component of his indwelling left ventricular assist device – is clearly visible.
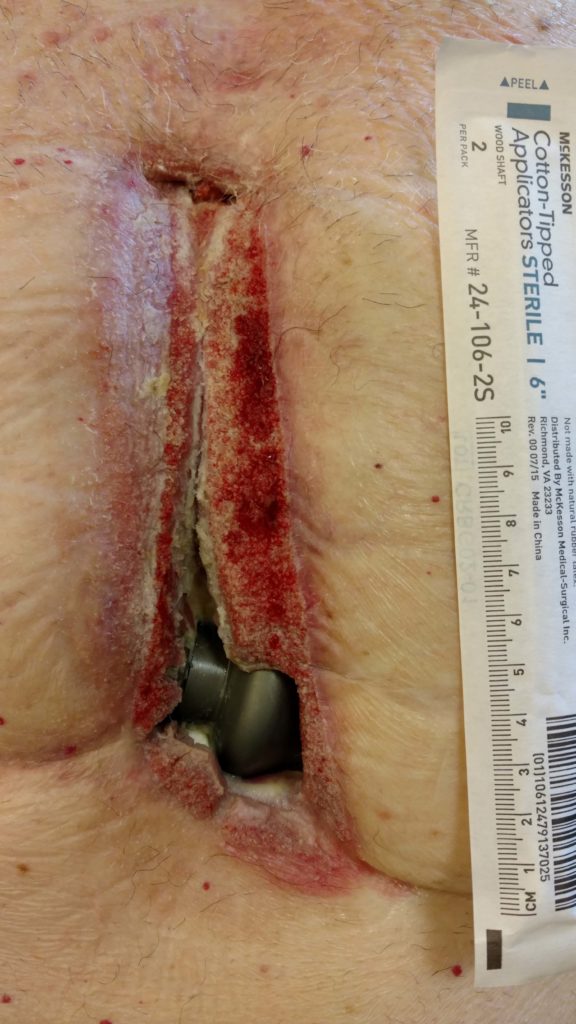
At the caudal end can be seen a 2 cm base of a an indwelling left ventricular assist device
The patient needed a new heart desperately, but replacing the infected mechanical pump that supported his own failing one threatened to unleash a lethal maelstrom of Staphylococcus aureus bacteria. He was denied heart transplant surgery by four hospitals. A fifth offered an experimental, Soviet-era-inspired treatment that not only subdued the slime-like biofilm but is also credited with enabling him to receive a new heart 4 weeks later in May 2018.
The patient attributes being alive and regaining strength almost a year later to the bold experimentation of infectious disease doctors and public health researchers at the University of California, San Diego. Over the past few years, the team has been helping seriously ill patients gain access to experimental treatments that use viruses, known as bacteriophages, to hunt down and kill bacteria with very strong specificity, including strains that are resistant to some of the most powerful antibiotics.
Spurred by the group’s successes – and some well publicised recent cases –bacteriophages are attracting growing awareness in both the general public and scientific community.
The lure of bacteriophages, or phages for short, is the hope that they might go at least some way in addressing a multitude of global health problems. These range from mitigating the impact of antimicrobial resistance (AMR) on the failure of modern medicines to treat infections, to sustaining infection-prone foreign implants, such as pacemakers and prosthetic hip joints, to selectively removing pathogens linked to food contamination and inflammatory bowel disease.
Phages share characteristics of the ultimate biodegradable “smart bomb” capable of eliminating a single bacterial target without affecting other beneficial bacteria. These spider-like viral predators, with transparent box-shaped heads that are 40 times smaller than a bacterium, are stimulated by their target pathogen to rapidly increase in number until the infection is cleared, even – as the San Diego patient’s case indicates – capable of thwarting the defensive nature of harmful biofilms. This bacteria-killing ability applies equally to drug-sensitive and drug-resistant bacteria. Importantly, phages are also able to elicit responses in bacteria that can result in other clinical benefits, such as reduced virulence and resensitisation to antimicrobial agents, even when the predatory powers of phages are overcome through phage-resistance. This can effectively “turn back the clock” on AMR by enabling bacteria to succumb to relatively narrow spectrum and older generation antibiotics.
While phages represent new and exciting tools for medical purposes, they have been deployed for more than three decades as biocontrol agents to tackle some of the biggest microbial threats to food production and safety. Their specificity makes them attractive tools to sanitise ready-to-eat foods, such as milk, vegetables and meat products. Phage-based products are now in mainstream use in North America to deactivate and eliminate problematic bacteria, offering significant public health benefits through improving animal and plant health, enhancing food safety, and reducing reliance on antimicrobials.
Negating these features are some inherent weaknesses. One of the key advantages of phages – their specificity – is also a major limitation. Each phage typically works on a single bacterium, and, often, on only a single subset or specific clone within that species. Before phages have any chance of clinical benefit, a practitioner needs to first identify the infectious agent, procure suitable phages that will safely kill the bacterial target, and then test, amplify, purify and prepare them for use. Besides requiring a more personalised approach to medicine that is more complicated than one that can rely on off-the-shelf products, consideration must also be given to ensuring that potentially therapeutic phages can get to and remain active in sufficient concentrations at the site of infection.
No broad-spectrum phage capable of infecting multiple bacterial species is known to exist or likely to be effective when widely used to treat polyclonal infections. Instead, polyvalent preparations composing diverse phages with distinct, but overlapping, host ranges have shown to be more efficacious in targeting a given species or subsets of species-specific strains. Multiple-phage cocktails can slow the adaptation process that leads to phage resistance in bacteria by pressuring the bacterium to develop mutations to evade several receptors simultaneously.
Such needs make bacteriophages more difficult and potentially slower to administer. This puts them at a distinct disadvantage to antibiotics, which are based on static, small molecules with clearly defined chemical structures. Antibiotics are often used empirically because of a spectrum of antibacterial activity that is typically broader, more reliable, and backed by rigorous scientific evidence gathered over decades. In contrast, no phage product has been approved for routine medical use by either the US Food and Drug Administration or the European Medicines Agency. A key reason for that is the dearth of data based on large scale, randomised controlled trials, which makes it difficult to gauge the therapeutic effect of phages, let alone how, where and over what duration they should be administered. Additionally, standards and regulations governing the definition of phage therapies and how they should be prepared for clinical use are lacking.
A key impediment to regulatory approval is the dynamic nature of phages and their interaction with bacteria. The two have been effectively at war for more than 3 billion years, leading to a co-evolution that persists today and will no doubt continue into the future. This makes the biological responses of phages to bacteria, as well as to the host’s immune system, difficult to predict without more study. Partly as a result of that, questions remain about their pharmacokinetics and pharmacodynamics. One fundamental concern centres on what happens to the genetic material of a phage once it attaches to a bacterial target.
Phages can be broadly categorised based on their activity inside a host bacterial cell. They are designated as “lytic” if they stimulate their host to virulently replicate and release more phage virions, or progeny, in self-replicating explosions that spread exponentially throughout a bacterial colony until the disease-causing bacteria are significantly reduced. Alternatively, they are designated as “temperate” if they stealthily or passively hide inside their bacterium. Only lytic phages can be applied therapeutically.
Temperate phages are a potential public health hazard, resembling the microbial equivalent of a “Trojan horse”. That is because they can integrate into the host genome or exist as extrachromosomal elements as so-called prophages that are replicated during normal bacterial cell division. These prophages can stay dormant in their host unperturbed until they are activated by environmental conditions or some other trigger unfavourable for growth. At this point, the prophage may excise itself from the bacterial genome and behave like a virulent phage.
Temperate phages may assist bacteria by acting as their “mules”, transferring DNA from one species of bacteria to another in a process known as transduction. The horizontal movement of such genetic material can facilitate the emergence of new bacterial variants with additional powers, such as reduced antibiotic sensitivity or heightened virulence. Examples of phage-mediated menaces include the foodborne Shiga toxin-producing EHEC O157:H7 strain of Escherichia coli that carries 18 prophages, the toxin-producing strains of cholera-causing Vibrio cholerae, and the AMR genes in S. aureus. Although contamination with temperate phages can be avoided, therapeutic phages require careful strain selection and propagation under controlled conditions.
Advances in synthetic biology may address such concerns through the engineering of phages lacking potentially hazardous features. Synthetic phages may also overcome some inherent limitations of natural phages, such as narrow host range and resistance development, and be patentable, providing intellectual property protection and thus improving their commercial prospects.
Currently, companies seeking to commercialise phage-based products for human therapeutic use are, in broad terms, pursuing one of two approaches to tackle the major causes of bacterial disease against which current therapies are ineffective, due largely to AMR or the production of biofilms.
One approach aims to produce standardised phage-based cocktails targeting a particular type of infection or selected pathogen resulting in an “off-the-shelf”-type of product that can mitigate an unmet, or poorly met, medical need. The alternative pathway seeks to produce unique, phage-based products that are individually formulated to target infection-causing bacteria isolated from patients on a case-by-case basis.
Clinicians and researchers in Australia are actively investigating phage-based treatments.
In 2018, there was a case reported of a 21-year-old man who had a stroke and was in a coma after a severe bloodstream infection overwhelmed his body. An artificial heart valve that replaced the patient’s own defective one years earlier had become infected with a bio-film-forming S. aureus that not only invaded the prosthetic device but was also constantly dispersing dangerous bacteria throughout the young man’s system. The infection was successfully cleared with antibiotics and 2 weeks of intravenous treatment using the phage product AB-SA01, an anti-S. aureus cocktail, enabling the infection-damaged valve to be successfully replaced. The investigational phage product was approved by the Therapeutic Goods Administration through a special emergency provision and made available via a compassionate access program that has now been used in more than a dozen such serious cases. The investigator acknowledged that the results were surprising but that without comparative studies, there was not enough evidence to confirm the results were due to the phage product.
Similar positive findings have been reported in a patient with cystic fibrosis for whom surgery and antibiotic therapy were unable to cure a debilitating biofilm. The positive outcome follows a phase 1 patient trial in which AB-SA01 phage therapy was found to be safe and demonstrated “activity” in nine patients with chronic rhinosinusitis treated in 2016.
These cases indicate that phages may play an important role in medicine as alternatives or adjuncts to antibiotics. The extent to which that role can expand beyond individual patient care to improving public health is much less clear. It is a question that scientists, doctors, medical authorities, public health practitioners, and phage manufacturers have been grappling with since phages were discovered in the UK and France just over a century ago.
Recent favorable publicity about successful individual cases has amplified the demand for an answer. That has spurred funding to support the requisite research, culminating in North America’s first centre for clinical phage research, which opened in San Diego in June 2018.
Ironically, phage successes reminiscent of the ones reported here and in California have been replicated throughout the past century – possibly numbering well into the tens of thousands.
Until recently, stories about such phage recipients have seldom, if ever, been published in the mainstream English language media because they occurred either behind “the Iron Curtain” during the Soviet era, or later in post-Soviet and East European nations, where clinical success using phage treatment was so routine as to be perceived as unremarkable. In any case, the technique was never subjected there to double-blinded randomised controlled trials – the gold standard for evaluating medical interventions.
The history of phages, however, has consistently shown a lack of self-reflective learning and discipline. Their use – and misuse – has pivoted on possibility and overzealousness, undermined by greed and, until recent decades, the lack of technology to support scientifically, systematically and rigorously gathered evidence. The only way the role of phages in public health can be conclusively established is through scientifically rigorous evidence. The global crisis posed by a post-antibiotic era means the world cannot afford to wait another decade until uncoordinated research provides, at best, qualified answers. Indications of the ability of phages to tackle multidrug-resistant infections and biofilms are sufficient to warrant urgent and careful study to determine the scope of their role in improving public health. Phage therapy is no silver bullet for overcoming the threat of AMR, but in the absence of any clear contenders, it must be properly explored.
This is an abridged version of research that helped earn Melbourne-based journalist Jason Gale a Master of Health Security degree at the University of Sydney in 2018.
The statements or opinions expressed in this article reflect the views of the authors and do not represent the official policy of the AMA, the MJA or InSight+ unless so stated.

 more_vert
more_vert
Since the journal publishes on April 1 only one year in seven, there should be a special edition, like the Christmas edition. I am looking forward to US media being taken in and reporting spoof papers from journals in the British lineage.