Researchers at the Frazer Institute, UQ, have developed an antibody that targets triple negative breast cancer — as well as other types of cancers — by helping natural killer cells to ‘see’ and kill cancer with fewer roadblocks.
InSight+ spoke with researchers, who recently published their work in Molecular Therapy.
The University of Queensland’s Associate Professor Fernando Guimaraes is an associate professor and group leader at the Translational Research Institute, within the Frazer Institute at UQ.
He said that the antibody they’ve developed recognises a unique part of the ROR1 protein, which is found on many aggressive cancers, but rarely on healthy cells.
“The antibody precisely targets cancer cells, helping the immune system kill cancer more effectively while aiming to spare healthy tissue,’’ said A/Prof. Guimaraes.
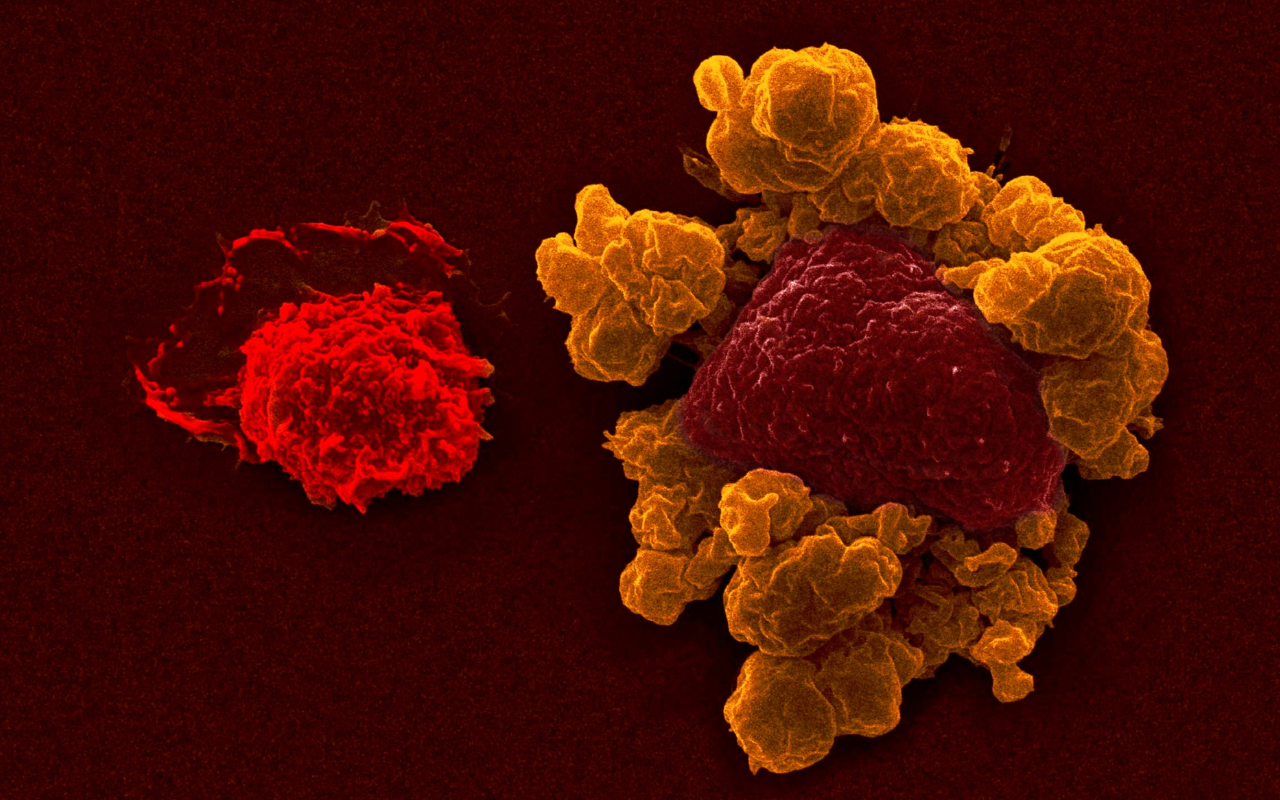
“Natural killer (NK) cells are immune cells responsible for patrolling our body and sensing cells that could become cancers, or that are infected by viruses, and proactively eliminate them before they become major issues,” said A/Prof. Guimaraes.
This research was a multi-disciplinary and translational collaboration, with work from a number of organisations, both Australian and international.
“NK cells have an inner potential of killing, which recently we have been observing that we can manipulate. We can make them better killers, or even tailor immunotherapy, trying to direct them with more efficiency to diseases they might be able to eliminate.”
“Our research is trying to give superpowers to the immune cells and direct them towards specific cancers.”
“It’s a tailored approach, to force NK cells to kill diseases that they might not necessarily ‘see.’ Because when the cancer transforms, they need to have minimum alterations to be visible to these cells. And using these new therapies, by showing these cancer cells with antibodies to the NK cells, we can enhance their ability to kill.”
“This can work for some cancers, not all. But some of the cancers we are pretty excited about, because they are naturally hard to cure.”

A multi-pronged approach
A/Prof. Guimaraes said that there are a few different ways to interfere with a cancer cell’s adaptation to the immune system.
“Something that we have recently been starting to understand is that the cancer cells do stop the immune response against them.”
“From the cancer microenvironment side, what are the cancer cells doing that are stopping this natural killer response?”
“We have two ways to get around that.”
“We can empower the natural killer cells to kill the cancer cells. And we can also shield these enemy cells, so that they don’t listen to the cancers when they tell them to not kill.”
“We published our research in Molecular Therapy a few weeks ago, having developed a new antibody that can flag specific cancer cells that express this oncofoetal receptor, ROR1. Which is one protein that is usually expressed on the cells when they are still stem cells.”
“When we are in foetal development, is when the receptor is usually expressed on the tissues. When the adult tissues are formed, that gene is no longer there.”
“But the cancer cells change a lot. There are so many mutations going on that they start to express things that they are not supposed to express, such as this foetal receptor. So, we can show this oncofoetal receptor to the natural killer cells with this antibody.”
“It’s preclinical work. We are not in clinical trials yet. We’re still defining the preclinical potential of this antibody to move to the next step, which hopefully could be a clinical option in the future.”
Targeting triple negative breast cancer
The research has targeted the antibody’s effectiveness in the treatment of triple negative breast cancer.
“Our team has studied the potential to treat triple negative breast cancers.”
“Triple negative breast cancer is the most challenging breast cancer. One of the reasons is that there isn’t a well-defined list of targets that we could use to target this specific breast cancer as we have for the other breast cancer categories.”
“The evidence that this specific receptor can be expressed on triple negative breast cancer in different models is very exciting, because it brings to light a way that we can target that cancer. And our tools seem to be highly applicable. That’s one of the reasons why we published a study — it made it the perfect rationale to investigate.”
“But there are other cancers that also could benefit from this targeting, such as ovarian cancers.”
“We are creating studies to try to assess what other cancers could be of interest. Some blood cancers could also benefit from our approach.”
A gentler treatment
The researchers say that this form of treatment is gentler, but not weaker.
“There is one exciting aspect of natural killer cell-based immunotherapy, particularly on transplantation settings,” A/Prof. Guimaraes said.
“If we take the natural killer cells from the blood, then we engineer them with molecular technologies, such as the one we published, to make them express chimeric antigen receptors (CARs). This process has been inspired by the chimeric antigen receptor T cells, CAR-T cells. There are already seven FDA-approved CAR-T cell therapies on the market for blood cancers.”
“The CAR-T cells are very efficient, but they do come with some limitations.”
“For example, they need to be patient-to-patient specific, because from a donor to another patient there is a high potential of transplantation issues, like graft-versus-host disease (GvHD). They currently carry toxicity risk as well, such as cytokine release syndrome (CRS), and potential neurotoxicity associated with their inflammatory reaction. “
“The evidence so far from early stage clinical trials shows that the natural killer cells-based immunotherapies don’t have those major toxicity issues.”
“Graft-versus-host disease hasn’t been observed when NK cells are going from a donor to a different patient. Also, there hasn’t been as much observed of this cytokine release syndrome, which is very exciting. So, what it means is that it is gentler, but it doesn’t mean weak. It means more controlled, or potentially less toxic.”
What is next in directing NK therapies?
“We need to define a more comprehensive safety study. There is already some safe evidence that suggests that our antibody would cause less toxicity, particularly in pro-killer cells-based immunotherapy,” A/Prof. Guimaraes said.
“NK cell-based approaches are believed to be less toxic than other therapies. They don’t really cause transplantation issues on the clinical trials data that we have today.”
“But we still need more data to solidify our conclusions. For these particular antibodies and CARs, we need to not only be safe, but also define their manufacturing pipeline.”
“Without funding, we don’t move this research forward. With the little funding we have, we make slow progress towards moving something that could benefit the patient’s life.”
A/Prof. Guimaraes is working on the future of immunotherapy at the Frazer Institute.
“We did have some translational industries contacting us to see the potential for partnering up, which is very exciting. We are in the process of discussions with them to see if we can move things forward.”
A/Prof. Guimaraes said that he is inspired by the Institute’s namesake, Emeritus Professor Ian Frazer, and Dr Jian Zhu, who discovered the Gardasil vaccine, which prevents HPV-induced cervical cancer. He continues to work with Professor Frazer, and the Institute runs immunotherapy focused teaching within postgraduate programs.
“Thirty years ago, oncologists did not believe that immunotherapy would do anything against cancer. It’s a mindset that has been changing over time, in the last decades. Today, immunotherapy is the front line of treatment for many diseases.”
This research was a multi-disciplinary and translational collaboration between UQ, Queensland Cyber Infrastructure Foundation, Mater Research Institute, Peter MacCallum Cancer Centre, Olivia Newton-John Cancer Research Institute, PUCPR in Brazil.
Becca Whitehead is a freelance journalist and health writer. She lives in Naarm and is a regular contributor to the MJA’s InSight+.
Subscribe to the free InSight+ weekly newsletter here. It is available to all readers, not just registered medical practitioners.

 more_vert
more_vert