The SARS-CoV-2 virus has caused enormous global healthcare challenges and further led to longer-term health complications for millions of infected individuals. Much remains to be learned about the virus, but one thing is clear: the SARS-CoV-2 virus can unleash widespread, systemic effects throughout the body due to its ability to cause damage to endothelial cells (termed endotheliitis). This process is associated with microvasculature dysfunction and is a major cause of the acute and chronic complications (Long-COVID-19) of the disease.
Stroke remains one of Australia’s most pressing health challenges. Each year, more than 50,000 Australians experience a stroke, making it a leading cause of adult disability and a major cause of death. While advances in clot-busting drugs (like tissue plasminogen activator, or t-PA) and mechanical thrombectomy save countless lives, outcomes are still sobering: more than half of survivors are left with long-term disability, and recurrence rates remain high.
Why? Part of the answer lies in the damage to the brain’s microcirculation, where vascular obstruction is hard to see and even harder to treat.
What we discovered
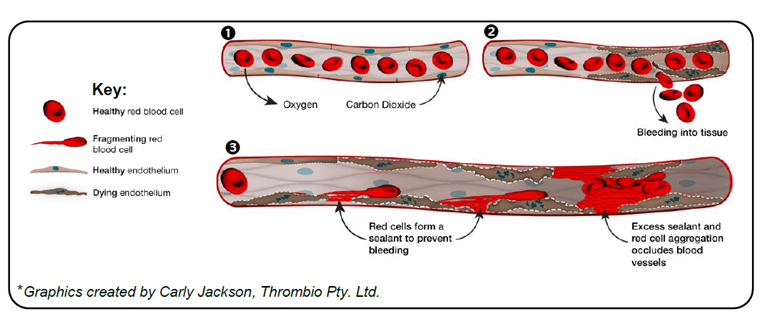
In studies published recently in Nature, our laboratory together with our international collaborators, have uncovered a surprising new mechanism leading to microvascular obstruction (See graphic). By examining autopsy samples from individuals dying from COVID-19, we identified an unexpected high rate of endothelial cell death in the microvasculature of major organs. The current dogma is that damage to the endothelium leads to a prothrombotic and proinflammatory state that exacerbates tissue injury. Remarkably, the majority of damaged and dying endothelial cells were not leading to the development of fibrin and platelet microclots. Nor were inflammatory cells present. Rather, dying endothelium triggered a previously unrecognised red blood cell (RBC) haemolytic process, that lead to the deposition of RBC membranes throughout the microvasculature. These damaged red cells become sticky and induce RBC aggregation, leading to extensive obstruction of capillaries (see graphic).
Importantly, we also showed that this process doesn’t occur in isolation. It’s a “two-hit” scenario. Platelet- and fibrin-rich microclots can form in arterioles, small arteries and veins, as well as post-capillary venules. This, in turn, is likely to worsen downstream hypoxia, exacerbating endothelial death and RBC damage in capillaries.
Our studies identified that the central cause of endothelial death and RBC haemolysis was ischaemia, and not surprisingly, tissue samples from non-COVID-19 patients with organ ischaemia experienced microvascular endothelial cell death and RBC haemolysis. Our new findings may provide important new insights into the dreaded ‘no-reflow’ phenomenon, in which heart attack and stroke patients’ symptoms can continue to worsen even after apparently successful large-artery reperfusion therapy.
Of course, every study has its limitations, and much of the mechanistic work was based on animal models and post-mortem human tissue. Translating these findings into the clinic will require a great deal more insight into which patients are at high risk of this new microvascular occlusion mechanism, and how to optimally prevent this process occurring in the first place.
The long arc of science
In many ways, our recent findings echo observations made more than 80 years ago (here, here and here). Pathologists in the 1940s and 50s described puzzling patterns in blood and tissue: abnormal “sludging” of red cells, odd shapes under the microscope, and mysterious aggregates forming around diseased tissue.
Early electron micrographs hinted at something unusual — cells clumping, membranes sticking, vessels inexplicably blocked. Yet at the time, the tools to probe these phenomena simply did not exist.
Those reports, published in journals such as Science during the post-war era, were prescient but incomplete. Without molecular techniques or advanced imaging, these scientists could only speculate on why red cells behaved this way, and their ideas faded into the margins of medical history.
COVID-19 brought these questions back into sharp focus. Autopsy samples revealed the same granular blockages that earlier generations had glimpsed but could not explain. With today’s technology, we can now understand the molecular mechanisms promoting endothelial cell death and RBC haemolysis.
In many ways, this is science coming full circle: old clues, new tools, and a clearer picture at last.
A new therapeutic horizon
Our team’s most recent discoveries have led us to explore new treatment strategies. We are developing a new antiplatelet therapy, called TBO-309, that has an unprecedented safety and efficacy profile in animal models and Phase 1 human trials (here, here and here). Unlike older drugs that indiscriminately thin the blood and raise the bleeding risk, TBO-309 modulates key platelet activation processes critical for pathological clot formation, whilst minimising the impact on the normal haemostatic function of platelets. By preventing platelet thrombus formation in large arteries, and reducing the development of microclots, TBO-309 has the potential to improve microvascular perfusion and prevent endothelial death and RBC haemolysis.
The Phase II STARS (NCT05363397) trial is currently evaluating the safety and efficacy of TBO-309 when used in combination with tPA in stroke patients. A second companion trial, Co-STARS (NCT06813651) will evaluate the safety and efficacy of TBO-309 at preventing stent thrombosis in patients experiencing a tandem occlusion stroke. If successful, TBO-309 could represent the first antiplatelet therapy that can be used currently with tPA in the hyperacute management of stroke.
What next?
For policymakers and health services, the challenge is to support the translation of these scientific insights into real-world therapies. That means sustained investment in investigator-led trials, Australian biotech innovation, and the infrastructure to deliver experimental drugs safely within our stroke units.
For patients, the message is urgent but also hopeful. Urgent, because every minute lost in stroke care still costs brain function. Hopeful, because science is now uncovering why recovery often stalls, and opening the door to new, more targeted treatments.
With STARS and Co-STARS underway, we are on track to generate first-in-human efficacy signals within the next 12 months, accelerating our path to partnerships with global pharmaceutical companies. If successful, TBO-309 could establish an entirely new standard of care — not only in stroke, but in other conditions where thrombosis and microvascular failure drives poor outcomes.
As someone who has spent a career chasing clots under the microscope, I see this as a moment of both reckoning and opportunity. We’ve spent decades focused on vascular highways; it’s time to clear the laneways and alleyways in our brains microvessels. If we can do that, we may finally shift the dial on stroke outcomes in Australia — and the world.
Professor Jackson is a NHMRC Investigator (Leadership level 3), a longstanding faculty member at Scripps Research (California, USA) and Honorary Visiting fellow at the University of Cambridge, UK and Florey Institute of Neuroscience and Mental Health (Victoria). He has established several Australian biotechnology companies focussing on the clinical development of novel antithrombotic therapeutics.
Professor Jackson is the Founder and Director of ThromBio Holdings Pty Ltd (ThromBio), the company that is developing the investigational anti-platelet therapy TBO-309.
The statements or opinions expressed in this article reflect the views of the authors and do not necessarily represent the official policy of the AMA, the MJA or InSight+ unless so stated.
Subscribe to the free InSight+ weekly newsletter here. It is available to all readers, not just registered medical practitioners.
If you would like to submit an article for consideration, send a Word version to mjainsight-editor@ampco.com.au.

 more_vert
more_vert